Use of the Decipher genomic classifier among men with prostate cancer in the United States
- PMID: 37525535
- PMCID: PMC10505256
- DOI: 10.1093/jncics/pkad052
Use of the Decipher genomic classifier among men with prostate cancer in the United States
Abstract
Background: Management of localized or recurrent prostate cancer since the 1990s has been based on risk stratification using clinicopathological variables, including Gleason score, T stage (based on digital rectal exam), and prostate-specific antigen (PSA). In this study a novel prognostic test, the Decipher Prostate Genomic Classifier (GC), was used to stratify risk of prostate cancer progression in a US national database of men with prostate cancer.
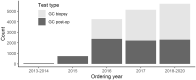
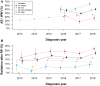
Methods: Records of prostate cancer cases from participating SEER (Surveillance, Epidemiology, and End Results) program registries, diagnosed during the period from 2010 through 2018, were linked to records of testing with the GC prognostic test. Multivariable analysis was used to quantify the association between GC scores or risk groups and use of definitive local therapy after diagnosis in the GC biopsy-tested cohort and postoperative radiotherapy in the GC-tested cohort as well as adverse pathological findings after prostatectomy.
Results: A total of 572 545 patients were included in the analysis, of whom 8927 patients underwent GC testing. GC biopsy-tested patients were more likely to undergo active active surveillance or watchful waiting than untested patients (odds ratio [OR] =2.21, 95% confidence interval [CI] = 2.04 to 2.38, P < .001). The highest use of active surveillance or watchful waiting was for patients with a low-risk GC classification (41%) compared with those with an intermediate- (27%) or high-risk (11%) GC classification (P < .001). Among National Comprehensive Cancer Network patients with low and favorable-intermediate risk, higher GC risk class was associated with greater use of local therapy (OR = 4.79, 95% CI = 3.51 to 6.55, P < .001). Within this subset of patients who were subsequently treated with prostatectomy, high GC risk was associated with harboring adverse pathological findings (OR = 2.94, 95% CI = 1.38 to 6.27, P = .005). Use of radiation after prostatectomy was statistically significantly associated with higher GC risk groups (OR = 2.69, 95% CI = 1.89 to 3.84).
Conclusions: There is a strong association between use of the biopsy GC test and likelihood of conservative management. Higher genomic classifier scores are associated with higher rates of adverse pathology at time of surgery and greater use of postoperative radiotherapy.In this study the Decipher Prostate Genomic Classifier (GC) was used to analyze a US national database of men with prostate cancer. Use of the GC was associated with conservative management (ie, active surveillance). Among men who had high-risk GC scores and then had surgery, there was a 3-fold higher chance of having worrisome findings in surgical specimens.
© The Author(s) 2023. Published by Oxford University Press.
Conflict of interest statement
James Proudfoot, Yang Liu, Xin Zhao, and Elai Davicioni are employees of Veracyte, Inc the makers of the Decipher test. Ashley Ross reports consulting fees as a medical advisor to Veracyte, Inc. The remaining co-authors declare no conflicts of interest.
Figures

References
-
- Dess RT, Suresh K, Zelefsky MJ, et al. Development and validation of a clinical prognostic stage group system for nonmetastatic prostate cancer using disease-specific mortality results from the International Staging Collaboration for Cancer of the Prostate. JAMA Oncol. 2020;6(12):1912-1920. doi: 10.1001/jamaoncol.2020.4922. - DOI - PMC - PubMed
-
- Jairath NK, Dal Pra A, Vince R Jr, et al. A systematic review of the evidence for the decipher genomic classifier in prostate cancer. Eur Urol. 2021;79(3):374-383. - PubMed
-
- Feng FY, Huang HC, Spratt DE, et al. Validation of a 22-gene genomic classifier in patients with recurrent prostate cancer: an ancillary study of the NRG/RTOG 9601 randomized clinical trial. JAMA Oncol 2021;7(4):544-552. doi: 10.1001/jamaoncol.2020.7671. Erratum in: JAMA Oncol. 2021;7(4):639. - DOI - PMC - PubMed
-
- Attard G, Parry M, Grist E, et al. Clinical testing of transcriptome-wide expression profiles in high-risk localized and metastatic prostate cancer starting androgen deprivation therapy: an ancillary study of the STAMPEDE abiraterone Phase 3 trial. Preprint. Res Sq. 2023;rs.3.rs-2488586. Published 2023 Feb 8. doi: 10.21203/rs.3.rs-2488586/v1. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
