Ciprofloxacin Derivative-Loaded Nanoparticles Synergize with Paclitaxel Against Type II Human Endometrial Cancer
- PMID: 37525558
- PMCID: PMC10828114
- DOI: 10.1002/smll.202302931
Ciprofloxacin Derivative-Loaded Nanoparticles Synergize with Paclitaxel Against Type II Human Endometrial Cancer
Abstract
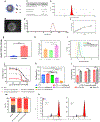
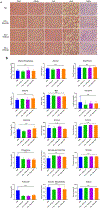
Combinations of chemotherapeutic agents comprise a clinically feasible approach to combat cancers that possess resistance to treatment. Type II endometrial cancer is typically associated with poor outcomes and the emergence of chemoresistance. To overcome this challenge, a combination therapy is developed comprising a novel ciprofloxacin derivative-loaded PEGylated polymeric nanoparticles (CIP2b-NPs) and paclitaxel (PTX) against human type-II endometrial cancer (Hec50co with loss of function p53). Cytotoxicity studies reveal strong synergy between CIP2b and PTX against Hec50co, and this is associated with a significant reduction in the IC50 of PTX and increased G2/M arrest. Upon formulation of CIP2b into PEGylated polymeric nanoparticles, tumor accumulation of CIP2b is significantly improved compared to its soluble counterpart; thus, enhancing the overall antitumor activity of CIP2b when co-administered with PTX. In addition, the co-delivery of CIP2b-NPs with paclitaxel results in a significant reduction in tumor progression. Histological examination of vital organs and blood chemistry was normal, confirming the absence of any apparent off-target toxicity. Thus, in a mouse model of human endometrial cancer, the combination of CIP2b-NPs and PTX exhibits superior therapeutic activity in targeting human type-II endometrial cancer.
Keywords: PEGylated polymeric nanoparticles; ciprofloxacin derivative; endometrial cancer; nanomedicine; paclitaxel.
© 2023 The Authors. Small published by Wiley‐VCH GmbH.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare that they have no competing interests.
Figures



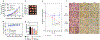

References
-
- Setiawan VW; Yang HP; Pike MC; McCann SE; Yu H; Xiang YB; Wolk A; Wentzensen N; Weiss NS; Webb PM; van den Brandt PA; van de Vijver K; Thompson PJ; Australian National Endometrial Cancer Study, G.; Strom BL; Spurdle AB; Soslow RA; Shu XO; Schairer C; Sacerdote C; Rohan TE; Robien K; Risch HA; Ricceri F; Rebbeck TR; Rastogi R; Prescott J; Polidoro S; Park Y; Olson SH; Moysich KB; Miller AB; McCullough ML; Matsuno RK; Magliocco AM; Lurie G; Lu L; Lissowska J; Liang X; Lacey JV Jr.; Kolonel LN; Henderson BE; Hankinson SE; Hakansson N; Goodman MT; Gaudet MM; Garcia-Closas M; Friedenreich CM; Freudenheim JL; Doherty J; De Vivo I; Courneya KS; Cook LS; Chen C; Cerhan JR; Cai H; Brinton LA; Bernstein L; Anderson KE; Anton-Culver H; Schouten LJ; Horn-Ross PL, Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2013, 31 (20), 2607–18. DOI 10.1200/JCO.2012.48.2596. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

