[Vaccination against malaria]
- PMID: 37525687
- PMCID: PMC10387304
- DOI: 10.48327/mtsi.v3i2.2023.325
[Vaccination against malaria]
Abstract
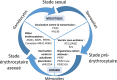
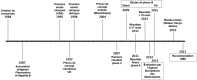
Vaccination against malaria is an old dream that reemerged in 2015 with the European Medicines Agency's favourable opinion on a first antimalarial vaccine, RTS,S/ AS01. Six years later, the World Health Organization (WHO) is advising a wide deployment of this vaccine in sub-Saharan Africa and in regions with high and moderate transmission where Plasmodium falciparum circulates. This follows favourable results from the pilot programme in Ghana, Kenya and Malawi involving over 800,000 children since 2019. This article addresses the objectives and main vaccine candidates targeting the different stages of parasite development, highlighting the progress and limitations of these different approaches. The RTS,S saga has been a milestone in vaccine development, with a first-generation vaccine recommended by the WHO for use in children over 5 months of age in sub-Saharan Africa and other areas of moderate to high transmission of P. falciparum malaria, in combination with other prevention measures. Research efforts continue to better understand the correlates of protection. With advances in vaccine platforms, new multi-antigen, multi-stage, and even multi-species approaches might emerge and brighten the horizon for malaria control.
L'espoir d'une vaccination contre le paludisme commence à se dessiner concrètement depuis 2015 avec l'avis favorable de l'Agence européenne du médicament sur un premier vaccin antipaludique, RTS,S/AS01. Six ans plus tard, l'Organisation mondiale de la santé (OMS) conseille un large déploiement de ce vaccin en Afrique subsaharienne et dans les régions à transmission forte et modérée où circule Plasmodium falciparum. Cette décision fait suite aux résultats favorables du programme pilote réalisé au Ghana, au Kenya et au Malawi sur plus de 800 000 enfants depuis 2019. Cet article décrit les objectifs et les principaux candidats vaccinaux ciblant les différents stades de développement du parasite, en soulignant les progrès et les limites de ces différentes approches. L’épopée RTS,S a été une étape clé dans le développement vaccinal, avec un vaccin de première génération recommandé par l'OMS chez les enfants de plus de 5 mois en Afrique subsaharienne et dans d'autres régions où la transmission du paludisme à P. falciparum est modérée ou forte, en association avec les autres mesures de prévention. Les efforts de recherche continuent pour mieux comprendre les corrélats de protection. Grâce aux progrès des plateformes vaccinales, de nouvelles approches multi-antigéniques, multistades, voire multi-espèces pourraient voir le jour et éclaircir l'horizon de la lutte contre le paludisme.
Keywords: Alphonse Laveran; Malaria; Plasmodium falciparum; Prevention; RTS,S/AS01; Sub-Saharan Africa; Vaccination; WHO.
Copyright © 2023 SFMTSI.
Figures
Similar articles
-
Safety and immunogenicity of RTS,S/AS01 malaria vaccine in infants and children with WHO stage 1 or 2 HIV disease: a randomised, double-blind, controlled trial.Lancet Infect Dis. 2016 Oct;16(10):1134-1144. doi: 10.1016/S1473-3099(16)30161-X. Epub 2016 Jul 7. Lancet Infect Dis. 2016. PMID: 27394191 Free PMC article. Clinical Trial.
-
Estimated impact of RTS,S/AS01 malaria vaccine allocation strategies in sub-Saharan Africa: A modelling study.PLoS Med. 2020 Nov 30;17(11):e1003377. doi: 10.1371/journal.pmed.1003377. eCollection 2020 Nov. PLoS Med. 2020. PMID: 33253211 Free PMC article.
-
Assessing the safety, impact and effectiveness of RTS,S/AS01E malaria vaccine following its introduction in three sub-Saharan African countries: methodological approaches and study set-up.Malar J. 2022 Apr 25;21(1):132. doi: 10.1186/s12936-022-04144-3. Malar J. 2022. PMID: 35468801 Free PMC article.
-
Policy uptake and implementation of the RTS,S/AS01 malaria vaccine in sub-Saharan African countries: status 2 years following the WHO recommendation.BMJ Glob Health. 2024 Apr 30;9(4):e014719. doi: 10.1136/bmjgh-2023-014719. BMJ Glob Health. 2024. PMID: 38688566 Free PMC article. Review.
-
[The malaria vaccine candidate RTS,S/AS is in phase III clinical trials].Ann Pharm Fr. 2010 Nov;68(6):370-9. doi: 10.1016/j.pharma.2010.07.002. Epub 2010 Oct 13. Ann Pharm Fr. 2010. PMID: 21073995 Review. French.
Cited by
-
[Malaria today].Med Trop Sante Int. 2023 May 15;3(2):mtsi.v3i2.2023.375. doi: 10.48327/mtsi.v3i2.2023.375. eCollection 2023 Jun 30. Med Trop Sante Int. 2023. PMID: 37525676 Free PMC article. French.
References
-
- Datoo MS, Natama HM, Somé A, Bellamy D, Traoré O, Rouamba T, Tahita MC, Ido NFA, Yameogo P, Valia D, Millogo A, Ouedraogo F, Soma R, Sawadogo S, Sorgho F, Derra K, Rouamba E, Ramos-Lopez F, Cairns M, Provstgaard-Morys S, Aboagye J, Lawrie A, Roberts R, Valéa I, Sorgho H, Williams N, Glenn G, Fries L, Reimer J, Ewer KJ, Shaligram U, Hill AVS, Tinto H. Efficacy and immunogenicity of R21/Matrix-M vaccine against clinical malaria after 2 years’ follow-up in children in Burkina Faso: a phase 1/2b randomised controlled trial. Lancet Infect Dis. 2022;22(12):1728–1736. doi: 10.1016/S1473-3099(22)00442-X. - DOI - PubMed
-
- Datoo MS, Natama MH, Somé A, Traoré O, Rouamba T, Bellamy D, Yameogo P, Valia D, Tegneri M, Ouedraogo F, Soma R, Sawadogo S, Sorgho F, Derra K, Rouamba E, Orindi B, Ramos Lopez F, Flaxman A, Cappuccini F, Kailath R, Elias S, Mukhopadhyay E, Noe A, Cairns M, Lawrie A, Roberts R, Valéa I, Sorgho H, Williams N, Glenn G, Fries L, Reimer J, Ewer KJ, Shaligram U, Hill AVS, Tinto H. Efficacy of a low-dose candidate malaria vaccine, R21 in adjuvant Matrix-M, with seasonal administration to children in Burkina Faso: a randomised controlled trial. Lancet. 2021;397(10287):1809–1818. doi: 10.1016/S0140-6736(21)00943-0. - DOI - PMC - PubMed
-
- OMS Malaria Vaccine Technology Roadmap. Organisation mondiale de la santé. 2013. Nov, p. 11 pages. www.who.int/publications/m/item/malaria-vaccine-technology-roadmap .
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
