Regulation of antigen-specific T cell infiltration and spatial architecture in multiple myeloma and premalignancy
- PMID: 37526080
- PMCID: PMC10378152
- DOI: 10.1172/JCI167629
Regulation of antigen-specific T cell infiltration and spatial architecture in multiple myeloma and premalignancy
Abstract
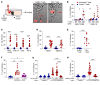
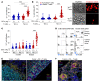
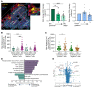
Entry of antigen-specific T cells into human tumors is critical for immunotherapy, but the underlying mechanisms are poorly understood. Here, we combined high-dimensional spatial analyses with in vitro and in vivo modeling to study the mechanisms underlying immune infiltration in human multiple myeloma (MM) and its precursor monoclonal gammopathy of undetermined significance (MGUS). Clustered tumor growth was a feature of MM but not MGUS biopsies, and this growth pattern was reproduced in humanized mouse models. MM biopsies exhibited intralesional as well as spatial heterogeneity, with coexistence of T cell-rich and T cell-sparse regions and the presence of areas of T cell exclusion. In vitro studies demonstrated that T cell entry into MM clusters was regulated by agonistic signals and CD2-CD58 interactions. Upon adoptive transfer, antigen-specific T cells localized to the tumor site but required in situ DC-mediated antigen presentation for tumor entry. C-type lectin domain family 9 member A-positive (CLEC9A+) DCs appeared to mark portals of entry for gradients of T cell infiltration in MM biopsies, and their proximity to T cell factor 1-positive (TCF1+) T cells correlated with disease state and risk status. These data illustrate a role for tumor-associated DCs and in situ activation in promoting the infiltration of antigen-specific T cells in MM and provide insights into spatial alterations in tumor/immune cells with malignant evolution.
Keywords: Adaptive immunity; Cancer immunotherapy; Hematology; Immunology.
Figures