Validated graft-specific biomarkers identify patients at risk for chronic graft-versus-host disease and death
- PMID: 37526081
- PMCID: PMC10378149
- DOI: 10.1172/JCI168575
Validated graft-specific biomarkers identify patients at risk for chronic graft-versus-host disease and death
Abstract
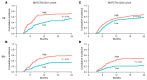
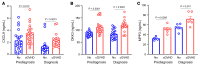
BACKGROUNDChronic graft-versus-host disease (cGVHD) is a serious complication of allogeneic hematopoietic cell transplantation (HCT). More accurate information regarding the risk of developing cGVHD is required. Bone marrow (BM) grafts contribute to lower cGVHD, which creates a dispute over whether risk biomarker scores should be used for peripheral blood (PB) and BM.METHODSDay 90 plasma proteomics from PB and BM recipients developing cGVHD revealed 5 risk markers that were added to 8 previous cGVHD markers to screen 982 HCT samples of 2 multicenter Blood and Marrow Transplant Clinical Trials Network (BMTCTN) cohorts. Each marker was tested for its association with cause-specific hazard ratios (HRs) of cGVHD using Cox-proportional-hazards models. We paired these clinical studies with biomarker measurements in a mouse model of cGVHD.RESULTSSpearman correlations between DKK3 and MMP3 were significant in both cohorts. In BMTCTN 0201 multivariate analyses, PB recipients with 1-log increase in CXCL9 and DKK3 were 1.3 times (95% CI: 1.1-1.4, P = 0.001) and 1.9 times (95%CI: 1.1-3.2, P = 0.019) and BM recipients with 1-log increase in CXCL10 and MMP3 were 1.3 times (95%CI: 1.0-1.6, P = 0.018 and P = 0.023) more likely to develop cGVHD. In BMTCTN 1202, PB patients with high CXCL9 and MMP3 were 1.1 times (95%CI: 1.0-1.2, P = 0.037) and 1.2 times (95%CI: 1.0-1.3, P = 0.009) more likely to develop cGVHD. PB patients with high biomarkers had increased likelihood to develop cGVHD in both cohorts (22%-32% versus 8%-12%, P = 0.002 and P < 0.001, respectively). Mice showed elevated circulating biomarkers before the signs of cGVHD.CONCLUSIONBiomarker levels at 3 months after HCT identify patients at risk for cGVHD occurrence.FUNDINGNIH grants R01CA168814, R21HL139934, P01CA158505, T32AI007313, and R01CA264921.
Keywords: Bone marrow transplantation; Transplantation.
Conflict of interest statement
Figures
References
-
- Williams KM, et al. National Institutes of Health consensus development project on criteria for Clinical Trials in chronic graft-versus-host disease: i. The 2020 Etiology and Prevention Working Group Report. Transplant Cell Ther. 2021;27(6):452–466. doi: 10.1016/j.jtct.2021.02.035. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

