Perioperative glycaemic control for people with diabetes undergoing surgery
- PMID: 37526194
- PMCID: PMC10392034
- DOI: 10.1002/14651858.CD007315.pub3
Perioperative glycaemic control for people with diabetes undergoing surgery
Abstract
Background: People with diabetes mellitus are at increased risk of postoperative complications. Data from randomised clinical trials and meta-analyses point to a potential benefit of intensive glycaemic control, targeting near-normal blood glucose, in people with hyperglycaemia (with and without diabetes mellitus) being submitted for surgical procedures. However, there is limited evidence concerning this question in people with diabetes mellitus undergoing surgery.
Objectives: To assess the effects of perioperative glycaemic control for people with diabetes undergoing surgery.
Search methods: For this update, we searched the databases CENTRAL, MEDLINE, LILACS, WHO ICTRP and ClinicalTrials.gov. The date of last search for all databases was 25 July 2022. We applied no language restrictions.
Selection criteria: We included randomised controlled clinical trials (RCTs) that prespecified different targets of perioperative glycaemic control for participants with diabetes (intensive versus conventional or standard care).
Data collection and analysis: Two authors independently extracted data and assessed the risk of bias. Our primary outcomes were all-cause mortality, hypoglycaemic events and infectious complications. Secondary outcomes were cardiovascular events, renal failure, length of hospital and intensive care unit (ICU) stay, health-related quality of life, socioeconomic effects, weight gain and mean blood glucose during the intervention. We summarised studies using meta-analysis with a random-effects model and calculated the risk ratio (RR) for dichotomous outcomes and the mean difference (MD) for continuous outcomes, using a 95% confidence interval (CI), or summarised outcomes with descriptive methods. We used the GRADE approach to evaluate the certainty of the evidence (CoE).
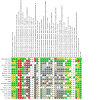
Main results: A total of eight additional studies were added to the 12 included studies in the previous review leading to 20 RCTs included in this update. A total of 2670 participants were randomised, of which 1320 were allocated to the intensive treatment group and 1350 to the comparison group. The duration of the intervention varied from during surgery to five days postoperative. No included trial had an overall low risk of bias. Intensive glycaemic control resulted in little or no difference in all-cause mortality compared to conventional glycaemic control (130/1263 (10.3%) and 117/1288 (9.1%) events, RR 1.08, 95% CI 0.88 to 1.33; I2 = 0%; 2551 participants, 18 studies; high CoE). Hypoglycaemic events, both severe and non-severe, were mainly experienced in the intensive glycaemic control group. Intensive glycaemic control may slightly increase hypoglycaemic events compared to conventional glycaemic control (141/1184 (11.9%) and 41/1226 (3.3%) events, RR 3.36, 95% CI 1.69 to 6.67; I2 = 64%; 2410 participants, 17 studies; low CoE), as well as those considered severe events (37/927 (4.0%) and 6/969 (0.6%), RR 4.73, 95% CI 2.12 to 10.55; I2 = 0%; 1896 participants, 11 studies; low CoE). Intensive glycaemic control, compared to conventional glycaemic control, may result in little to no difference in the rate of infectious complications (160/1228 (13.0%) versus 224/1225 (18.2%) events, RR 0.75, 95% CI 0.55 to 1.04; P = 0.09; I2 = 55%; 2453 participants, 18 studies; low CoE). Analysis of the predefined secondary outcomes revealed that intensive glycaemic control may result in a decrease in cardiovascular events compared to conventional glycaemic control (107/955 (11.2%) versus 125/978 (12.7%) events, RR 0.73, 95% CI 0.55 to 0.97; P = 0.03; I2 = 44%; 1454 participants, 12 studies; low CoE). Further, intensive glycaemic control resulted in little or no difference in renal failure events compared to conventional glycaemic control (137/1029 (13.3%) and 158/1057 (14.9%), RR 0.92, 95% CI 0.69 to 1.22; P = 0.56; I2 = 38%; 2086 participants, 14 studies; low CoE). We found little to no difference between intensive glycaemic control and conventional glycaemic control in length of ICU stay (MD -0.10 days, 95% CI -0.57 to 0.38; P = 0.69; I2 = 69%; 1687 participants, 11 studies; low CoE), and length of hospital stay (MD -0.79 days, 95% CI -1.79 to 0.21; P = 0.12; I2 = 77%; 1520 participants, 12 studies; very low CoE). Due to the differences within included studies, we did not pool data for the reduction of mean blood glucose. Intensive glycaemic control resulted in a mean lowering of blood glucose, ranging from 13.42 mg/dL to 91.30 mg/dL. One trial assessed health-related quality of life in 12/37 participants in the intensive glycaemic control group, and 13/44 participants in the conventional glycaemic control group; no important difference was shown in the measured physical health composite score of the short-form 12-item health survey (SF-12). One substudy reported a cost analysis of the population of an included study showing a higher total hospital cost in the conventional glycaemic control group, USD 42,052 (32,858 to 56,421) compared to the intensive glycaemic control group, USD 40,884 (31.216 to 49,992). It is important to point out that there is relevant heterogeneity between studies for several outcomes. We identified two ongoing trials. The results of these studies could add new information in future updates on this topic.
Authors' conclusions: High-certainty evidence indicates that perioperative intensive glycaemic control in people with diabetes undergoing surgery does not reduce all-cause mortality compared to conventional glycaemic control. There is low-certainty evidence that intensive glycaemic control may reduce the risk of cardiovascular events, but cause little to no difference to the risk of infectious complications after the intervention, while it may increase the risk of hypoglycaemia. There are no clear differences between the groups for the other outcomes. There are uncertainties among the intensive and conventional groups regarding the optimal glycaemic algorithm and target blood glucose concentrations. In addition, we found poor data on health-related quality of life, socio-economic effects and weight gain. It is also relevant to underline the heterogeneity among studies regarding clinical outcomes and methodological approaches. More studies are needed that consider these factors and provide a higher quality of evidence, especially for outcomes such as hypoglycaemia and infectious complications.
Trial registration: ClinicalTrials.gov NCT00433251 NCT00995501 00370643 NCT00524472 00107601 NCT00609986 NCT00460499 NCT00220987 NCT01643382 NCT01211730 NCT00443599 NCT01565941 NCT01187329 NCT00788242 NCT01227148 https://doi.org/10.1186/s12871-019-0918-0 NCT03526536 NCT01886365 NCT00899483 NCT01528189 NCT02746432 NCT03314272 NCT034474666 NCT02032953.
Copyright © 2023 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
The authors declare that they do not have any conflicts of interest.
Figures























Update of
-
Perioperative glycaemic control for diabetic patients undergoing surgery.Cochrane Database Syst Rev. 2012 Sep 12;(9):CD007315. doi: 10.1002/14651858.CD007315.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2023 Aug 1;8:CD007315. doi: 10.1002/14651858.CD007315.pub3. PMID: 22972106 Updated.
References
References to studies included in this review
Abdelmalak 2013 {published and unpublished data}
-
- Abdelmalak BB, Bonilla, Mascha EJ, Maheshwari A, Tang WH, You MJ, et al. Dexamethasone, light anaesthesia, and tight glucose control(DeLiT) randomized controlled trial. British Journal of Anaesthesia 2013;111(2):209-21. - PubMed
-
- NCT00433251. The effects of corticosteroids, glucose control, and depth-of-anaesthesia on perioperative inflammation and morbidity form major non-cardiac surgery (dexamethasone, light anesthesia and tight glucose control (DeLiT Trial)). clinicaltrials.gov/ct2/show/NCT00433251 (first received 15 October 2009).
-
- NCT00995501. The effects of coroticosteroids, glucose control, and depth-of-anesthesia on perioperative inflammation and morbidity from major non-cardiac surgery (dexamethasone, light anesthesia and tight glucose control (DeLiT Trial)). clinicaltrials.gov/ct2/show/NCT00995501 (first received 15 October 2009).
Cao 2010 {published data only}
-
- Cao SG, Ren JA, Shen B, Chen D, Zhou YB, Li JS. Intensive versus conventional insulin in type 2 diabetes patients undergoing D2 gastrectomy for gastric cancer: a randomized controlled trial. World Journal of Surgery 2011;35(1):85-92. - PubMed
Chan 2009 {published and unpublished data}
-
- NCT00370643. Glucose control in open heart surgery. clinicaltrials.gov/ct2/show/NCT00370643 (first received 1 September 2006).
De La Rosa 2008 {published and unpublished data}
Desai 2012 {published data only}
-
- Desai SP, Henry LL, Holmes SD, Hunt SL, Martin CT, Hebsur S, et al. Strict versus liberal target range for perioperative glucose in patients undergoing coronary artery bypass grafting: a prospective randomized controlled trial. Journal of Thoracic and Cardiovascular Surgery 2012;143(2):318-25. - PubMed
Duncan 2018 {published and unpublished data}
-
- NCT00524472. Outcomes study of hyperinsulinemic glucose control in cardiac surgery. clinicaltrials.gov/ct2/show/NCT00524472 (first received 3 September 2007).
Gandhi 2007 {published data only}
-
- Gandhi GY, Nuttall GA, Abel M, Mullany CJ, Schaff HV, O'Brien PC, et al. Intensive intraoperative insulin therapy versus conventional glucose management during cardiac surgery. Annals of Internal Medicine 2007;146(4):233-43. - PubMed
-
- NCT00282698. Outcomes with tight control of hyperglycemia in cardiac surgery patients. clinicaltrials.gov/ct2/show/NCT00282698 (first received 27 January 2006).
Glucontrol 2009 {published and unpublished data}
-
- NCT00107601. Glucontrol study: comparing the effects of two glucose control regimens by insulin in intensive care unit patients. clinicaltrials.gov/ct2/show/NCT00107601 (first received 6 April 2005).
-
- Preiser J-C, Devos P, Ruiz-Santana S, Mélot C, Annane D, Groeneveld J, et al. A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Medicine 2009;35(10):1738-48. - PubMed
Hermayer 2012 {published data only}
-
- Hermayer KL, Egidi MF, Finch NJ, Baliga P, Lin A, Kettinger L, et al. A randomized controlled trial to evaluate the effect of glycemic control on renal transplantation outcomes. Journal of Clinical Endocrinology and Metabolism 2012;97:4399–406. - PubMed
Lazar 2004 {published data only (unpublished sought but not used)}
-
- Lazar HL, Chipkin SR, Fitzgerald CA, Bao Y, Cabral H, Apstein CS. Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events. Circulation 2004;109(12):1497-502. - PubMed
Lazar 2011 {published data only}
-
- Lazar HL, McDonnell MM, Chipkin S, Fitzgerald C, Bliss C, Cabral H. Effects of aggressive versus moderate glycemic control on clinical outcomes in diabetic coronary artery bypass graft patients. Annals of Surgery 2011;254(3):458-64. [NCT00460499] - PubMed
-
- NCT00460499. Improving outcomes in diabetic patients during CABG surgery by omptimizing glycemic control. clinicaltrials.gov/ct2/show/NCT00460499 (first received 16 April 2007).
Li 2006 {published data only (unpublished sought but not used)}
NICE SUGAR 2009 {published and unpublished data}
-
- Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, et al, NICE-SUGAR Study Investigators. Intensive versus conventional glucose control in critically ill patients. New England Journal of Medicine 2009;360(13):1283-97. - PubMed
-
- NCT00220987. Normoglycaemia in intensive care evaluation and survival using glucose algorithm regulation (NICE-SUGAR STUDY). clinicaltrials.gov/ct2/show/NCT00220987 (first received 22 September 2005).
Parekh 2016 {published data only}
-
- NCT01643382. Hyperglycemia in renal transplantation (HiRT). clinicaltrials.gov/ct2/show/NCT01643382 (first received 18 July 2012).
Rassias 1999 {published data only}
-
- Rassias AJ, Marrin CAS, Arruda J, Whalen PK, Beach M, Yeager MP. Insulin infusion improves neutrophil function in diabetic cardiac surgery patients. Anesthesia and Analgesia 1999;88(5):1011-6. - PubMed
Subramaniam 2009 {published and unpublished data}
-
- Subramaniam B, Panzica PJ, Novack V, Mahmood F, Matyal R, Mitchell JD, et al. Continuous perioperative insulin infusion decreases major cardiovascular events in patients undergoing vascular surgery: a prospective, randomized trial. Anesthesiology 2009;110(5):970-7. - PubMed
Umpierrez 2015 {published and unpublished data}
-
- NCT01361594. Intensive insulin therapy in patients undergoing coronary artery bypass surgery (CABG). clinicaltrials.gov/ct2/show/NCT01361594 (first received 31 December 2014).
-
- Umpierrez G, Cardona S, Pasquel F, Jacobs S, Peng L, Unigwe M, et al. Randomized controlled trial of intensive versus conservative glucose control in patients undergoing coronary arterybypass graft surgery: GLUCO-CABG trial. Diabetes Care 2015;38(9):1665–72. [DOI: 10.2337/dc15-0303] - DOI - PMC - PubMed
Wahby 2016 {published data only}
-
- Wahby EA, Abo Elnasr MM, Eissa MI, Mahmoud SM. Perioperative glycemic control in diabetic patients undergoingcoronary artery bypass graft surgery. Journal of the Egyptian Society of Cardio-Thoracic Surgery 2016;24:143-9. [DOI: ]
Wallia 2017 {published and unpublished data}
-
- NCT01211730. Study of glycemic control on liver transplantation outcomes. clinicaltrials.gov/ct2/show/NCT01211730 (first received 29 September 2010).
-
- Wallia A, Schmidt K, Oakes DJ, Pollack T, Welsh N, Kling-Colson S, et al. Glycemic control reduces infections in post–liver transplant patients: results of a prospective, randomized study. Journal of clinical Endocrinology and Metabolism 2017;102:451–9. [DOI: 10.1210/jc.2016-3279] - DOI - PMC - PubMed
References to studies excluded from this review
Abdelmalak 2019 {published and unpublished data}
-
- Abdelmalak BB, You J, Kurz A, Kot M, Bralliar T, Remzi FH, et al. The effects of dexamethasone, light anesthesia, and tight glucose control on postoperative fatigue and quality of life after major noncardiac surgery: a randomized trial. Journal of Clinical Anesthesia 2019;55:83-91. [DOI: ] - PubMed
Agus 2014 {published and unpublished data}
-
- NCT00443599. SPECS: safe pediatric euglycemia in cardiac surgery (SPECS). clinicaltrials.gov/ct2/show/results/NCT00443599 (first received 6 March 2007).
Agus 2017 {published and unpublished data}
-
- NCT01565941. Heart and lung failure - pediatric insulin titration trial (HALF-PINT). clinicaltrials.gov/ct2/show/NCT01565941 (first received 29 March 2012).
Asida 2013 {published data only}
-
- Asida SM, Atalla MMM, Gad GS, Aisa KM, Mohamed Hs. Effect of perioperative control of blood glucose level patient’s outcome after anesthesia for cardiac surgery. Egyptian Journal of Anaesthesia 2013;29:71-6.
Cao 2013 {published data only}
Chuah 2015 {published data only}
-
- Chuah LL, Miras AD, Papamargaritis D, Jackson SN, Olbers T, le Roux CW. Impact of perioperative management of glycemia in severely obese diabetic patients undergoing gastric bypass surgery. Surgery for Obesity and Related Diseases 2015;11(3):578-84. - PubMed
Cinotti 2014 {published data only}
-
- Cinotti R, Ichai C, Orban JC, Kalfon P, Feuillet F, Roquilly A, et al. Effects of tight computerized glucose control on neurological outcome in severely brain injured patients: a multicenter sub-group analysis of the randomized-controlled open-label CGAO-REA study. Critical Care (London, England) 2014;18(5):498. - PMC - PubMed
Duncan 2015 {published data only}
Ellenberger 2018 {published data only}
-
- Ellenberger C, Sologashvili T, Kreienbuhl L, Cikirikcioglu M, Diaper J, Licker M. Myocardial protection by glucose-insulin-potassium in moderate- to high-risk patients undergoing elective on-pump cardiac surgery: a randomized controlled trial. Anesthesia and Analgesia 2018;126(4):1133-41. - PubMed
Giakoumidakis 2013 {published data only}
-
- Giakoumidakis K, Eltheni R, Patelarou E, Theologou S, Patris V, Michopanou N, et al. Effects of intensive glycemic control on outcomes of cardiac surgery. Heart and Lung 2013;42(2):146-51. - PubMed
Gupta 2020 {published data only}
Hsu 2012 {published data only}
Huang 2013 {published data only}
-
- Huang QX, Lou FC, Wang P, , Liu Q, Wang K, Zhang L, et al. Basal insulin therapy strategy is superior to premixed insulin therapy in the perioperative period blood glucose management. Chinese Medical Journal 2013;126(21):4030-6. - PubMed
Kalfon 2014 {published data only}
-
- Kalfon P, Giraudeau B, Ichai C, Guerrini A, Brechot N, Cinotti R, et al, Group, Cgao-Rea Study. Tight computerized versus conventional glucose control in the ICU: a randomized controlled trial. Intensive Care Medicine 2014;40(1):171-81. - PubMed
Krishna 2019 {published data only}
-
- Parthasarathy Arun, Handattu Mahabalesware Krishna. Comparative study between insulin bolus regimen and glucose insulin infusion regimen on effectiveness of intraoperative blood glucose control in patients with type 2 diabetes mellitus undergoing non-cardiac surgery. Journal of Anaesthesiology 2019;27(2):110-14. [DOI: ]
Kumar 2020 {published and unpublished data}
Kurnaz 2017 {published data only}
-
- Kurnaz P, Sungur Z, Camci E, Sivrikoz N, Orhun G, Senturk M, et al. The effect of two different glycemic management protocols on postoperative cognitive dysfunction in coronary artery bypass surgery. Revista Brasileira de Anestesiologia 2017;67(3):258-65. - PubMed
Laiq 2015 {published data only}
-
- Laiq N, Khan S, Ahmed H, Aslam S, Khan RA. The effects of glycaemic control in cardiac patients undergoing CABG surgery. Journal of Medical Sciences (Peshawar, Print) 2015;23(1):21-5.
LÜ 2019 {published data only}
-
- Lü S, Huang X, Mao J, Li Lifen, Zhao Z. Application of intensive blood glucose management in HCC patients associated with diabetes during perioperative period of radiofrequency ablation. Journal of Interventional Radiology 2019;28:582-5. [DOI: 10.3969 / j.issn.1008-794X.2019.06.018]
Makino 2019 {published data only}
-
- Makino H, Tanaka A, Asakura K, Koezuka R, Tochiya M, Ohata Y, et al. Addition of low-dose liraglutide to insulin therapy is useful for glycaemic control during the peri-operative period: effect of glucagon-like peptide-1 receptor agonist therapy on glycaemic control in patients undergoing cardiac surgery (GLOLIA study). Diabetic Medicine 2019;36(12):1621-8. [DOI: 10.1111/dme.14084] - DOI - PubMed
Mikaeili 2012 {published data only}
-
- Mikaeili H, Yazdchi M, Barazandeh F, Ansarin K. Euglycemic state reduces the incidence of critical illness polyneuropathy and duration of ventilator dependency in medical intensive care unit. Bratislavske Lekarske Listy 2012;113(10):616-9. - PubMed
Mohod 2019 {published and unpublished data}
Mularski 2012 {published data only}
NCT00394303 {published data only}
-
- NCT00394303. Tight intra-operative glucose control during coronary artery bypass surgery. http://clinicaltrials.gov/show/NCT00394303 (accessed 30 July 2012).
NCT00487162 {published data only}
-
- NCT00487162. The association between peri-operative hyperglycemia and major morbidity and mortality. http://clinicaltrials.gov/show/NCT00487162 (accessed 30 July 2012).
NCT03526536 {published data only}
-
- NCT03526536. Pilot: Insulin sensitivity/management in hyperglycemic patients in perioperative period ESRD/Non-ESRD. https://clinicaltrials.gov/ct2/show/NCT03526536 (first received 16 May 2018). [CLINICALTRIALS.GOV: NCT03526536]
Okabayashi 2014 {published data only}
-
- Okabayashi T, Shima Y, Sumiyoshi T, Kozuki A, Tokumaru T, Liyama T, et al. Intensive versus intermediate glucose control in surgical intensive care unit patients. Diabetes Care 2014;37(6):1516-24. - PubMed
Pasquel 2020 {published data only}
-
- Pasquel FJ, Lansang MC, Khowaja A, Urrutia MA, Cardona S, Albury B, et al. A randomized controlled trial comparing glargine U300 and glargine U100 for the inpatient management of medicine and surgery patients with type 2 diabetes: Glargine U300 Hospital Trial. Diabetes Care 2020;43:1242-8. - PMC - PubMed
Pezzella 2014 {published data only}
-
- A Thomas Pezzella, Sari D Holmes, Graciela Pritchard, Alan M Speir, Niv Ad. Impact of Perioperative Glycemic Control Strategy on Patient Survival After Coronary Bypass Surgery [Impact of Perioperative Glycemic Control Strategy on Patient Survival After Coronary Bypass Surgery]. The Annals of Thoracic Surgery May 22 2014;98:1281-1285. [DOI: 10.1016/j.athoracsur.2014.05.067] [PMID: ] - DOI - PubMed
Polderman 2017 {published data only}
-
- Polderman JA, Ma XL, Eshuis WJ, Hollmann MW, DeVries JH, Preckel B, et al. Efficacy of continious intravenous glucose monitoring in perioperative glycaemic control: a randomized controlled study. British Journal of Anaesthesia 2017;118(2):264-75. - PubMed
Punke 2014 {published data only}
-
- Punke MA, Goepfert MS, Kluge S, Reichenspurner H, Goetz AE, Reuter DA. Perioperative glycemic control with a computerized algorithm versus conventional glycemic control in cardiac surgical patients undergoing cardiopulmonary bypass with blood cardioplegia. Journal of Cardiothoracic and Cascular Anesthesia 2014;28(5):1273-7. - PubMed
Qu 2012 {published data only}
-
- Qu CZ, Zhang RY, Xiang K F. Blood glucose control in the perioperative stage of cardiac value replacement influences levels of blood lactic acid. Chinese Journal of Tissue Engineering Research 2012;16(53):9955-9.
Ramírez‐Cáceres 2019 {published data only}
-
- César Ramírez-Cáceres, Estrella Uzcátegui Paz, Ricardo Lozano Hérnandez. Protocol for fast postoperative improvement (FPOI) in gastrointestinal surgery [Protocolo de rapida mejoria posoperatoria ( RAMPO) en cirugía gastrointestinal ]. cirugia y cirujanos 2018;87:151-7. - PubMed
Rujirojindakul 2014 {published data only}
-
- Rujirojindakul P, Liabsuetrakul T, McNeil E, Chanchayanon T, Wasinwong W, Oofuvong M, et al. Safety and efficacy of intensive intraoperative glycaemic control in cardiopulmonary bypass surgery: a randomised trial. Acta Anaesthesiologica Scandinavica 2014;58(5):588-96. - PubMed
Santana‐Santos 2019 {published and unpublished data}
-
- Eduesley Santana-Santos, Patricia Hatanaka Kanke, Rita de Cássia Almeida Vieira, Larissa Bertacchini de Oliveira, Renata Eloah de Lucena Ferretti-Rebustin, et al. Impact of intensive glycemic control on acute renal injury: a randomized clinical trial. Acta Paul Enferm 2019;32(6):592-9. [DOI: ]
Schroeder 2012 {published data only}
-
- Schroeder JE, Liebergall M, Raz I, Egleston R, Ben Sussan G, Peyser A, et al. Benefits of a simple glycaemic protocol in an orthopaedic surgery ward: a randomized prospective study. Diabetes/Metabolism Research and Reviews 2012;28(1):71-5. - PubMed
Tohya 2018 {published data only}
Wang 2017 {published data only}
Welsh 2016 {published data only}
References to studies awaiting assessment
Hweidi 2021 {published data only}
Imran‐ul‐hassan 2021 {published data only (unpublished sought but not used)}
-
- Syed Imran-ul-hassan, Sharyar, Muhammad Rashid, Maryam Liaqat, Javairia Saleem, Taiba Zulfiqar1. Outcome of strict peri-operative glycemic control in diabetic patients following open heart surgery. Med. Forum 2021;32(7):43-7.
NCT00899483 {published data only}
-
- NCT00899483. Can enhanced glycaemic control in type II diabetics improve myocardial protection during coronary artery bypass grafting?. https://clinicaltrials.gov/ct2/show/NCT00899483 (first received 12 May 2009). [CLINICALTRIAL.GOV: NCT00899483]
NCT03474666 {published data only}
-
- NCT03474666. Glycemic control and surgical site infection incidence among liver transplantation recipients. https://clinicaltrials.gov/ct2/show/NCT03474666 (first received 22 March 2018). [CLINICALTRIALS.GOV: NCT03474666]
Zadeh 2016 {published data only}
-
- Zadeh FJ, Ghazi Nour M. A study on the outcomes of modified tight glucose control for the management of glycemic control in diabetic patients undergoing cardiac surgery. Journal of Pharmacy Research 2016;10(11):764-70.
References to ongoing studies
NCT02032953 {published data only}
-
- NCT02032953. Enhancing the anabolic effect of perioperative nutrition with insulin while maintaining normoglycemia. https://clinicaltrials.gov/ct2/show/NCT02032953 (first received 10 January 2014).
NCT04742023 {published data only}
-
- NCT04742023. Post-operative complications and graft survival with conventional versus continuous glucose monitoring in patients with diabetes mellitus undergoing renal transplantation. https://clinicaltrials.gov/ct2/show/NCT04742023 (first received 5 February 2021).
Additional references
Abdelmalak 2011
-
- Abdelmalak B, Maheshwari A, Kovaci B, Marscha EJ, Cywinski JB, Kurz A, et al. Validation of the DeLIT Trial intravenous insulin infusion algorithm for intraoperative glucose control in noncardiac surgery: a randomized controlled trial.. Canadian Journal of Anaesthesia 2011;58(7):606-16.. [DOI: 10.1007/s12630-011-9509-3] - DOI - PubMed
ADA 2003
-
- Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 2003;26(Suppl 1):S5-20. - PubMed
ADA 2010
ADA 2021
-
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes. Diabetes Care 1 January 2021;44 (supplement(Supplement_1):15-33. [DOI: ]
AHRQ 2001
Albacker 2008
Alberti 1998
-
- Alberti KM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part I: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetic Medicine 1998;15(7):539-53. - PubMed
Altman 2003
Arabi 2008
-
- Yaseen M Arabi, Ousama C Dabbagh, Hani M Tamim, Abdullah A Al-Shimemeri, Ziad A Memish, Samir H Haddad, Sofia J Syed, Hema R Giridhar, Asgar H Rishu, Mouhamad O Al-Daker, Salim H Kahoul, Riette J Britts, Maram H Sakkijha. Intensive versus conventional insulin therapy: a randomized controlled trial in medical and surgical critically ill patients. Critical Care Medicine December 2008;36(12):3190-7. [DOI: ] - PubMed
Bell 2013
-
- Bell ML, McKenzie JE. Designing psycho-oncology randomised trials and cluster randomised trials: variance components and intra-cluster correlation of commonly used psychosocial measures. Psycho-oncology 2013;22:1738-47. - PubMed
Bilotta 2009
Boutron 2014
-
- Boutron I, Altman DG, Hopewell S, Vera-Badillo F, Tannock I, Ravaud P. Impact of spin in the abstracts of articles reporting results of randomized controlled trials in the field of cancer: the SPIIN randomized controlled trial. Journal of Clinical Oncology 2014;32(36):4120-6. - PubMed
Buch 2011
-
- Buch MH, Aletaha D, Emery P, Smolen JS. Reporting of long-term extension studies: lack of consistency calls for consensus. Annals of the Rheumatic Diseases 2011;70(6):886-90. - PubMed
Cardona 2017
-
- Saumeth Cardona, Francisco J pasquel, Maya Fayman, Limin Peng, Sol Jacobs, Priyathama Vellanki, Jef Weaver, Michael Halkos, Robert A Guyton, Vinod H Thouorani, Guillermo E Umpierrez. Hospitalization costs and clinical outcomes in CABG patients treated with intensive insulin therapy. Journal of Diabetes and its Complications 20 January 2017;31:742-747. [DOI: ] - PubMed
Carvalho 2011
-
- George Carvalho, Patricia Pelletier, Turki Albacker, Kevin Lachapelle, Denis R Joanisse, Roupen Hatzakorzian, Ralph Lattermann, Hiroaki Sato, André Marette, Thomas Schricker. Cardioprotective effects of glucose and insulin administration while maintaining normoglycemia (GIN therapy) in patients undergoing coronary artery bypass grafting. The Journal of Clinical Endocrinology & Metabolism May 2011;96(5):1469-77. [DOI: 10.1210/jc.2010-1934.] - DOI - PubMed
Cochrane 2022
-
- Cochrane. How CENTRAL is created. www.cochranelibrary.com/central/central-creation (accessed 17 June 2022).
CONSORT 2016
-
- Moher D, Schulz KF, Altman D, CONSORT Group (Consolidated Standards of Reporting Trials). The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA 2001;285(15):1987-1991. - PubMed
Corbett 2014
-
- Corbett MS, Higgins JP, Woolacott NF. Assessing baseline imbalance in randomised trials: implications for the Cochrane risk of bias tool. Research Synthesis Methods 2014;5(1):79-85. - PubMed
Deeks 2019
-
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. In: Deeks JJ, Higgins JPT, Altman DG, editors(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021.
Drayton 2022
Furnary 1999
-
- Furnary AP, Zerr KJ, Grunkemeier GL, Starr A. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Annals of Thoracic Surgery 1999;67(2):352-60; discussion 360-2. [MEDLINE: ] - PubMed
Gandhi 2005
-
- Gandhi GY, Nuttall GA, Abel MD, Mullany CJ, Schaff HV, Williams BA, et al. Intraoperative hyperglycemia and perioperative outcomes in cardiac surgery patients. Mayo Clinic Proceedings 2005;80(7):862-6. [MEDLINE: ] - PubMed
Golden 1999
-
- Golden SH, Peart-Vigilance C, Kao WH, Brancati FL. Perioperative glycemic control and the risk of infectious complications in a cohort of adults with diabetes. Diabetes Care 1999;22(9):1408-14. [MEDLINE: ] - PubMed
GRADEproGDT 2015 [Computer program]
-
- GRADEpro GDT: GRADEpro Guideline Development Tool. Hamilton (ON): McMaster University and Evidence Prime,, 2015. gradepro.org..
He 2007
-
- He W, Zhang TY, Zhou H, Li T, Zhao JY, Zhao D, et al. Impact of intensive insulin therapy on surgical critically ill patients. Chinese Journal of Surgery 2007;45(15):1052-4. [PMID: ] - PubMed
Higgins 2002
-
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;21:1539-58. - PubMed
Higgins 2003
Higgins 2019a
-
- Higgins JP, Li T, Deeks JJ (editors). Chapter 6: Choosing effect measures and computing estimates of effect.. In: Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019.
Higgins 2019b
-
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. . In: Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019.
Hoffmann 2014
-
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. - PubMed
Hoffmann 2017
-
- Hoffmann TC, Oxman AD, Ioannidis JP, Moher D, Lasserson TJ, Tovey DI, et al. Enhancing the usability of systematic reviews by improving the consideration and description of interventions. BMJ 2017;358:j2998. - PubMed
Hozo 2005
Hróbjartsson 2013
-
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and nonblinded assessors. Canadian Medical Association Journal 2013;185(4):E201-11. - PMC - PubMed
International Hypoglycaemia Study Group 2019
Jin 2020
Jones 2015
Kang 2018
Kirdemir 2008
-
- Kirdemir P, Yildirim V, Kiris I, Gulmen S, Kuralay E, Ibrisim E, et al. Does continuous insulin therapy reduce postoperative supraventricular tachycardia incidence after coronary artery bypass operations in diabetic patients. Journal of Cardiothorac Vascular Anesthesia 2008;22:383-387. - PubMed
Kirkham 2010
Lefebvre 2022
-
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf MI, et al, Cochrane Information Retrieval Methods Group. Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Marshall 2018
Mathieu 2009
-
- Mathieu S, Boutron I, Moher D, Altman DG, Ravaud P. Comparison of registered and published primary outcomes in randomized controlled trials. JAMA 2009;302:977-84. - PubMed
Meader 2014
Megan 2012
-
- Megan B, Pickering RM, Weatherall M. Design, objectives, execution and reporting of published open-label extension studies. Journal of Evaluation in Clinical Practice 2012;18(2):209-15. - PubMed
Mitchell 2006
-
- Imogen Mitchell, Emma Knight, Jelena Gissane, Rohit Tamhane, Rao Kolli, I Anne Leditschke, Rinaldo Bellomo, Simon Finfer, Australian and New Zealand Intensive Care Society Clinical Trials Group. A phase II randomised controlled trial of intensive insulin therapy in general intensive care patients. Critical Care Resuscitation December 2006 ;8(4):289-93. [PMID: ] - PubMed
Page 2020
-
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews [The PRISMA 2020 statement: an updated guideline for reporting systematic reviews]. BMJ 2021;372(71):1-9. [DOI: doi: 10.1136/bmj.n71] - PMC - PubMed
Pomposelli 1998
-
- Pomposelli JJ, Baxter JK 3rd, Babineau TJ, Pomfret EA, Driscoll DF, Forse RA, et al. Early postoperative glucose control predicts nosocomial infection rate in diabetic patients. Journal of Parenteral and Enteral Nutrition 1998;22(2):77-81. [MEDLINE: ] - PubMed
Review Manager 2020 [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Sathya 2013
-
- Sathya B, Davis R, Taveira T, Whitlatch H, Wu WC. Intensity of peri-operative glycaemic control and postoperative outcomes in patients with diabetes: a meta-analysis. Diabetes Res Clin Pract October 2013;102:8-15. - PubMed
Scherer 2007
Schünemann 2019
-
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the confidence in or quality of the evidence. . In: Cochrane Handbook for Systematic Reviews of Interventions version 6.0 . Cochrane, 2019.
Seaquist 2013
-
- Seaquist ER, Anderson J, Childs B, Cryer P, Dagogo-Jack S, Fish L, Heller SR, Rodriguez H, Rosenzweig J, Vigersky R. Hypoglycemia and diabetes: a report of a workgroup of the Diabetes Association and the Endocrine Society. Diabetes Care May 2013;36(5):1384-1395. [DOI: 10.2337/dc12-2480] [PMID: ] - DOI - PMC - PubMed
Smiley 2006
-
- Smiley DD, Umpierrez GE. Perioperative glucose control in the diabetic or nondiabetic patient. Southern Medical Journal 2006;99(6):580-9; quiz 590-1. [MEDLINE: ] - PubMed
Sterne 2011
-
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. - PubMed
Van den Berghe 2001
-
- Greet Van den Berghe, Pieter Wouters, Frank Weekers, Charles Verwaest, Frans Bruyninckx, Miet Schetz, Dirk Vlasselaers, Patrick Ferdinande, Peter Lauwers, Roger Bouillon. Intensive Insulin Therapy in Critically Ill Patients. N Engl J Med 8 November 2001 ;345:1359-1367. [DOI: 10.1056/NEJMoa011300] - DOI - PubMed
Wang 2008
-
- Wang QG, Lu LF, Zhou YB, Cao SG, Wang DS, Lv L. Effects of intensive insulin therapy on insulin resistance and serum proteins aTer radical gastrectomy.. Chinese Journal of Gastrointestinal Surgery 2008;11(5):444-7. [PMID: ] - PubMed
WHO 1999
-
- World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications. In: Report of a WHO Consultation. Part 1: Diagnosis and Classification of Diabetes Mellitus. Geneva: World Health Organization, 1999:1-59.
WHO 2021
-
- World Health Organization. Improving diabetes outcomes for all, a hundred years on from the discovery of insulin: report of the Global Diabetes Summit. Vol. Geneva: World Health Organization; 2021. Licence: CC BY-NC-SA 3.0 IGO. Geneva: World Health Organization , 2021. [LICENCE: CC BY-NC-SA 3.0 IGO.]
Wong 2006
References to other published versions of this review
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

