Fine-grained functional parcellation maps of the infant cerebral cortex
- PMID: 37526293
- PMCID: PMC10393291
- DOI: 10.7554/eLife.75401
Fine-grained functional parcellation maps of the infant cerebral cortex
Abstract
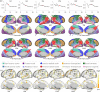
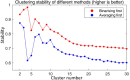
Resting-state functional MRI (rs-fMRI) is widely used to examine the dynamic brain functional development of infants, but these studies typically require precise cortical parcellation maps, which cannot be directly borrowed from adult-based functional parcellation maps due to the substantial differences in functional brain organization between infants and adults. Creating infant-specific cortical parcellation maps is thus highly desired but remains challenging due to difficulties in acquiring and processing infant brain MRIs. In this study, we leveraged 1064 high-resolution longitudinal rs-fMRIs from 197 typically developing infants and toddlers from birth to 24 months who participated in the Baby Connectome Project to develop the first set of infant-specific, fine-grained, surface-based cortical functional parcellation maps. To establish meaningful cortical functional correspondence across individuals, we performed cortical co-registration using both the cortical folding geometric features and the local gradient of functional connectivity (FC). Then we generated both age-related and age-independent cortical parcellation maps with over 800 fine-grained parcels during infancy based on aligned and averaged local gradient maps of FC across individuals. These parcellation maps reveal complex functional developmental patterns, such as changes in local gradient, network size, and local efficiency, especially during the first 9 postnatal months. Our generated fine-grained infant cortical functional parcellation maps are publicly available at https://www.nitrc.org/projects/infantsurfatlas/ for advancing the pediatric neuroimaging field.
Keywords: functional connectivity; functional parcellation; human; infant brain; neuroscience.
© 2023, Wang et al.
Conflict of interest statement
FW, HZ, ZW, DH, ZZ, JG, LW, WL, GL No competing interests declared
Figures









Update of
References
-
- Dennis EL, Jahanshad N, McMahon KL, de Zubicaray GI, Martin NG, Hickie IB, Toga AW, Wright MJ, Thompson PM. Development of brain structural connectivity between ages 12 and 30: a 4-Tesla diffusion imaging study in 439 adolescents and adults. NeuroImage. 2013;64:671–684. doi: 10.1016/j.neuroimage.2012.09.004. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

