Longitudinal Follow-Up of Participants With Tobacco Exposure and Preserved Spirometry
- PMID: 37526720
- PMCID: PMC10394572
- DOI: 10.1001/jama.2023.11676
Longitudinal Follow-Up of Participants With Tobacco Exposure and Preserved Spirometry
Abstract
Importance: People who smoked cigarettes may experience respiratory symptoms without spirometric airflow obstruction. These individuals are typically excluded from chronic obstructive pulmonary disease (COPD) trials and lack evidence-based therapies.
Objective: To define the natural history of persons with tobacco exposure and preserved spirometry (TEPS) and symptoms (symptomatic TEPS).
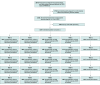
Design, setting, and participants: SPIROMICS II was an extension of SPIROMICS I, a multicenter study of persons aged 40 to 80 years who smoked cigarettes (>20 pack-years) with or without COPD and controls without tobacco exposure or airflow obstruction. Participants were enrolled in SPIROMICS I and II from November 10, 2010, through July 31, 2015, and followed up through July 31, 2021.
Exposures: Participants in SPIROMICS I underwent spirometry, 6-minute walk distance testing, assessment of respiratory symptoms, and computed tomography of the chest at yearly visits for 3 to 4 years. Participants in SPIROMICS II had 1 additional in-person visit 5 to 7 years after enrollment in SPIROMICS I. Respiratory symptoms were assessed with the COPD Assessment Test (range, 0 to 40; higher scores indicate more severe symptoms). Participants with symptomatic TEPS had normal spirometry (postbronchodilator ratio of forced expiratory volume in the first second [FEV1] to forced vital capacity >0.70) and COPD Assessment Test scores of 10 or greater. Participants with asymptomatic TEPS had normal spirometry and COPD Assessment Test scores of less than 10. Patient-reported respiratory symptoms and exacerbations were assessed every 4 months via phone calls.
Main outcomes and measures: The primary outcome was assessment for accelerated decline in lung function (FEV1) in participants with symptomatic TEPS vs asymptomatic TEPS. Secondary outcomes included development of COPD defined by spirometry, respiratory symptoms, rates of respiratory exacerbations, and progression of computed tomographic-defined airway wall thickening or emphysema.
Results: Of 1397 study participants, 226 had symptomatic TEPS (mean age, 60.1 [SD, 9.8] years; 134 were women [59%]) and 269 had asymptomatic TEPS (mean age, 63.1 [SD, 9.1] years; 134 were women [50%]). At a median follow-up of 5.76 years, the decline in FEV1 was -31.3 mL/y for participants with symptomatic TEPS vs -38.8 mL/y for those with asymptomatic TEPS (between-group difference, -7.5 mL/y [95% CI, -16.6 to 1.6 mL/y]). The cumulative incidence of COPD was 33.0% among participants with symptomatic TEPS vs 31.6% among those with asymptomatic TEPS (hazard ratio, 1.05 [95% CI, 0.76 to 1.46]). Participants with symptomatic TEPS had significantly more respiratory exacerbations than those with asymptomatic TEPS (0.23 vs 0.08 exacerbations per person-year, respectively; rate ratio, 2.38 [95% CI, 1.71 to 3.31], P < .001).
Conclusions and relevance: Participants with symptomatic TEPS did not have accelerated rates of decline in FEV1 or increased incidence of COPD vs those with asymptomatic TEPS, but participants with symptomatic TEPS did experience significantly more respiratory exacerbations over a median follow-up of 5.8 years.
Conflict of interest statement
Figures


References
-
- Global Initiative for Chronic Obstructive Lung Disease . Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2023 report. Accessed July 9, 2023. https://goldcopd.org/wp-content/uploads/2022/12/GOLD-2023-ver-1.1-2Dec20...
-
- Global Initiative for Chronic Obstructive Lung Disease . Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2021 report. Accessed July 9, 2023. https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25...

