Patient-Reported Outcomes in People with Type 2 Diabetes Receiving Tirzepatide in the SURPASS Clinical Trial Programme
- PMID: 37526908
- PMCID: PMC10570242
- DOI: 10.1007/s13300-023-01451-z
Patient-Reported Outcomes in People with Type 2 Diabetes Receiving Tirzepatide in the SURPASS Clinical Trial Programme
Abstract
Introduction: Tirzepatide, a novel glucose-dependent insulinotropic polypeptide and glucagon-like peptide 1 receptor agonist, is approved for glycaemic control for people with type 2 diabetes (T2D). The SURPASS-1 to -5 clinical trials assessed the efficacy of once weekly tirzepatide (5, 10 and 15 mg) versus placebo or active comparators (semaglutide 1 mg, insulin degludec and insulin glargine) in T2D. We evaluated patient-reported outcomes (PROs) that measured overall quality of life (QoL), treatment satisfaction and weight-related attributes across the five SURPASS studies.
Methods: PRO instruments utilised at baseline and primary timepoint (40 weeks for SURPASS-1, -2 and -5; 52 weeks for SURPASS-3 and -4) or early termination visit were EQ-5D-5L (SURPASS-1 to -5); Impact of Weight on Self-Perceptions (SURPASS-1 to -5); Ability to Perform Physical Activities of Daily Living (SURPASS-1 to -5); Diabetes Treatment Satisfaction Questionnaire (SURPASS-2 to -5); and Impact of Weight on Quality of Life-Lite Clinical Trials Version (SURPASS-2 only).
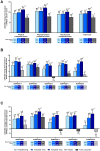
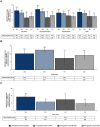
Results: Across all five studies at week 40/52, tirzepatide improved patients' QoL measured by general health and weight-related PROs over the comparator. Generally, higher doses of tirzepatide resulted in greater increases in PRO scores.
Conclusion: Overall, tirzepatide produced significant health and weight-related QoL improvements versus comparators in the five SURPASS studies.
Clinical trial registration: SURPASS-1: NCT03954834; SURPASS-2: NCT03987919; SURPASS-3: NCT03882970; SURPASS-4: NCT03730662; SURPASS-5: NCT04039503.
Keywords: Health-related quality of life patient-reported outcomes; Quality of life; SURPASS; Tirzepatide; Type 2 diabetes; Weight-related quality of life.
Plain language summary
Tirzepatide is the first glucose-dependent insulinotropic polypeptide and glucagon-like peptide 1 receptor agonist approved for the treatment of people with type 2 diabetes. The SURPASS-1 to -5 clinical trials evaluated the efficacy and safety of tirzepatide (5, 10 and 15 mg) compared with placebo or active comparators (including semaglutide 1 mg and basal insulins) in people with type 2 diabetes. We evaluated other outcomes reported by patients that measured overall quality of life, treatment satisfaction and weight-related attributes across the five SURPASS studies.Five validated questionnaires were completed by patients at the beginning and end of the clinical trials, which was after 40 weeks for SURPASS-1, -2 and -5 and after 52 weeks for SURPASS-3 and -4, or when the person left the trial if this was before the official end. These questionnaires were EQ-5D-5L (SURPASS-1 to -5); Impact of Weight on Self-Perceptions (SURPASS-1 to -5); Ability to Perform Physical Activities of Daily Living (SURPASS-1 to -5); Diabetes Treatment Satisfaction Questionnaire (SURPASS-2 to -5); and Impact of Weight on Quality of Life-Lite Clinical Trials Version (SURPASS-2 only).Across all five studies, treatment with tirzepatide resulted in greater improvements in people’s quality of life at the end of the study compared with placebo or treatment with the comparators. Generally, higher doses of tirzepatide resulted in greater increases in questionnaire scores than lower doses of tirzepatide.Overall, tirzepatide 5, 10 or 15 mg treatment resulted in significant health- and weight-related quality of life improvements versus comparators in the five SURPASS studies.
© 2023. The Author(s).
Conflict of interest statement
Kristina S Boye, Vivian Thuyanh Thieu, Hélène Sapin, Clare J Lee, Laura Fernández Landó, Katelyn Brown, Ross Bray, Russell J Wiese, Hiren Patel, Ángel Rodríguez and Maria Yu are full-time employees and stockholders of Eli Lilly and Company.
Figures
References
-
- United States Food and Drug Administration. Prescribing information: MOUNJARO (tirzepatide) injection, for subcutaneous use. Rockville, MD: FDA; 2022. p. 1–28. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215866s000lbl.pdf. Accessed 5 Sept 2022.
-
- European Commission. Mounjaro tirzepatide. Summary of product characteristics. Brussels: EC; 2022. p. 1–87. https://ec.europa.eu/health/documents/community-register/2022/2022091515.... Accessed 6 Oct 2022.
-
- Pharmaceuticals and Medical Devices Agency (PMDA). Manjaro tirzepatide. General list entry. https://www.pmda.go.jp/PmdaSearch/iyakuDetail/GeneralList/24994A8. Accessed 20 Oct 2022.
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

