Race and Treatment Outcomes in Patients With Metastatic Castration-Sensitive Prostate Cancer: A Secondary Analysis of the SWOG 1216 Phase 3 Trial
- PMID: 37526936
- PMCID: PMC10394570
- DOI: 10.1001/jamanetworkopen.2023.26546
Race and Treatment Outcomes in Patients With Metastatic Castration-Sensitive Prostate Cancer: A Secondary Analysis of the SWOG 1216 Phase 3 Trial
Abstract
Importance: Black patients present with more aggressive disease and experience higher mortality than White patients with prostate cancer. Race and social determinants of health influence prostate cancer-specific mortality and overall survival (OS); however, in a previous trial, Black patients did not have inferior outcomes compared with White patients, possibly because of equitable access to care available in a clinical trial setting.
Objective: To compare differences in survival outcomes of patients with metastatic castration-sensitive prostate cancer (mCSPC) by race in a phase 3 trial with a large proportion of Black patients.
Design, setting, and participants: This secondary analysis of patient-level data of a prospective phase 3 randomized clinical trial included patients with newly diagnosed mCSPC enrolled between March 1, 2013, and July 15, 2017. Analysis was conducted between December 2022 and February 2023.
Interventions: Patients receiving androgen deprivation therapy were randomized (1:1) to receive either orteronel 300 mg orally twice daily (experimental group) or bicalutamide 50 mg orally daily (control group).
Main outcomes and measures: OS, with progression-free survival (PFS) as a secondary end point.
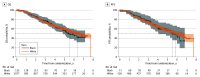
Results: Among 1313 participants, 135 (10%) identified as Black and 1077 (82%) as White, with an equal racial distribution between groups. Black patients were younger (median [IQR] age, 65.8 [60-70] vs 68.4 [62.5-74.1] years; P = .001) and had a higher median (IQR) baseline prostate-specific antigen response rate than White patients (54.7 [19.8-222.0] vs 26.7 [9.2-96.0] ng/mL; P < .001). At a median follow-up of 4.9 years, Black and White patients had similar median PFS (2.3 years; 95% CI, 1.8-1.4 years vs 2.9 years; 95% CI, 2.5-3.3 years; P = .71) and OS (5.5 years; 95% CI, 4.8-NR vs 6.3 years; 95% CI, 5.7-NR; P = .65). The multivariable analysis confirmed similar PFS and OS after adjusting for known prognostic factors. No interaction between race and treatment was observed.
Conclusions and relevance: In this secondary analysis of a randomized clinical trial studying androgen deprivation therapy with first- or second-generation androgen receptor pathway inhibitors, both Black and White patients demonstrated similar OS and PFS. Equitable access to care may reduce historical differences in outcomes between Black and White patients with advanced prostate cancer.
Trial registration: ClinicalTrials.gov Identifier: NCT01809691.