Repeated mild traumatic brain injury triggers pathology in asymptomatic C9ORF72 transgenic mice
- PMID: 37527465
- PMCID: PMC11046056
- DOI: 10.1093/brain/awad264
Repeated mild traumatic brain injury triggers pathology in asymptomatic C9ORF72 transgenic mice
Abstract
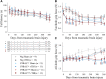
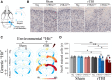
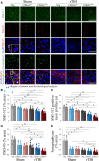
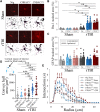
Frontotemporal dementia (FTD) and amyotrophic lateral sclerosis (ALS) are fatal neurodegenerative diseases that represent ends of the spectrum of a single disease. The most common genetic cause of FTD and ALS is a hexanucleotide repeat expansion in the C9orf72 gene. Although epidemiological data suggest that traumatic brain injury (TBI) represents a risk factor for FTD and ALS, its role in exacerbating disease onset and course remains unclear. To explore the interplay between traumatic brain injury and genetic risk in the induction of FTD/ALS pathology we combined a mild repetitive traumatic brain injury paradigm with an established bacterial artificial chromosome transgenic C9orf72 (C9BAC) mouse model without an overt motor phenotype or neurodegeneration. We assessed 8-10 week-old littermate C9BACtg/tg (n = 21), C9BACtg/- (n = 20) and non-transgenic (n = 21) mice of both sexes for the presence of behavioural deficits and cerebral histopathology at 12 months after repetitive TBI. Repetitive TBI did not affect body weight gain, general neurological deficit severity, nor survival over the 12-month observation period and there was no difference in rotarod performance, object recognition, social interaction and acoustic characteristics of ultrasonic vocalizations of C9BAC mice subjected to repetitive TBI versus sham injury. However, we found that repetitive TBI increased the time to the return of the righting reflex, reduced grip force, altered sociability behaviours and attenuated ultrasonic call emissions during social interactions in C9BAC mice. Strikingly, we found that repetitive TBI caused widespread microglial activation and reduced neuronal density that was associated with loss of histological markers of axonal and synaptic integrity as well as profound neuronal transactive response DNA binding protein 43 kDa mislocalization in the cerebral cortex of C9BAC mice at 12 months; this was not observed in non-transgenic repetitive TBI and C9BAC sham mice. Our data indicate that repetitive TBI can be an environmental risk factor that is sufficient to trigger FTD/ALS-associated neuropathology and behavioural deficits, but not paralysis, in mice carrying a C9orf72 hexanucleotide repeat expansion.
Keywords: C9orf72; amyotrophic lateral sclerosis; frontotemporal dementia; neurodegeneration; transactive response DNA binding protein 43 kDa (TDP-43).
© The Author(s) 2023. Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
The authors report no competing interests.
Figures


References
-
- Boeve BF, Boxer AL, Kumfor F, Pijnenburg Y, Rohrer JD. Advances and controversies in frontotemporal dementia: Diagnosis, biomarkers, and therapeutic considerations. Lancet Neurol. 2022;21:258–272. - PubMed
-
- Burrell JR, Halliday GM, Kril JJ, et al. The frontotemporal dementia-motor neuron disease continuum. Lancet. 2016;388:919–931. - PubMed
-
- Ratnavalli E, Brayne C, Dawson K, Hodges JR. The prevalence of frontotemporal dementia. Neurology. 2002;58:1615–1621. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

