Hepatic levels of S-adenosylmethionine regulate the adaptive response to fasting
- PMID: 37527658
- PMCID: PMC10432853
- DOI: 10.1016/j.cmet.2023.07.002
Hepatic levels of S-adenosylmethionine regulate the adaptive response to fasting
Abstract
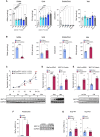
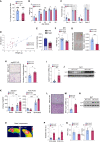
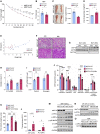
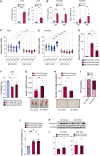
There has been an intense focus to uncover the molecular mechanisms by which fasting triggers the adaptive cellular responses in the major organs of the body. Here, we show that in mice, hepatic S-adenosylmethionine (SAMe)-the principal methyl donor-acts as a metabolic sensor of nutrition to fine-tune the catabolic-fasting response by modulating phosphatidylethanolamine N-methyltransferase (PEMT) activity, endoplasmic reticulum-mitochondria contacts, β-oxidation, and ATP production in the liver, together with FGF21-mediated lipolysis and thermogenesis in adipose tissues. Notably, we show that glucagon induces the expression of the hepatic SAMe-synthesizing enzyme methionine adenosyltransferase α1 (MAT1A), which translocates to mitochondria-associated membranes. This leads to the production of this metabolite at these sites, which acts as a brake to prevent excessive β-oxidation and mitochondrial ATP synthesis and thereby endoplasmic reticulum stress and liver injury. This work provides important insights into the previously undescribed function of SAMe as a new arm of the metabolic adaptation to fasting.
Keywords: S-adenosylmethionine; adipose tissue; caloric restriction; endoplasmic reticulum stress; fasting; liver; methionine adenosyltransferase a1; mitochondria-associated-membranes; phosphatidylethanolamine methyltransferase; β-oxidation.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests M.L.M.-C. advises for Mitotherapeutix.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

