Repeat polymorphisms underlie top genetic risk loci for glaucoma and colorectal cancer
- PMID: 37527660
- PMCID: PMC10528368
- DOI: 10.1016/j.cell.2023.07.002
Repeat polymorphisms underlie top genetic risk loci for glaucoma and colorectal cancer
Abstract
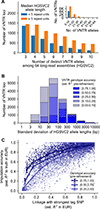
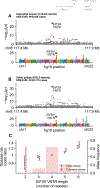
Many regions in the human genome vary in length among individuals due to variable numbers of tandem repeats (VNTRs). To assess the phenotypic impact of VNTRs genome-wide, we applied a statistical imputation approach to estimate the lengths of 9,561 autosomal VNTR loci in 418,136 unrelated UK Biobank participants and 838 GTEx participants. Association and statistical fine-mapping analyses identified 58 VNTRs that appeared to influence a complex trait in UK Biobank, 18 of which also appeared to modulate expression or splicing of a nearby gene. Non-coding VNTRs at TMCO1 and EIF3H appeared to generate the largest known contributions of common human genetic variation to risk of glaucoma and colorectal cancer, respectively. Each of these two VNTRs associated with a >2-fold range of risk across individuals. These results reveal a substantial and previously unappreciated role of non-coding VNTRs in human health and gene regulation.
Keywords: GWAS; VNTR; colorectal cancer; expression and splicing quantitative trait loci; genetic associations; genomic structural variation; glaucoma; imputation; tandem repeat; variable numbers of tandem repeats.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

