Trends in antihypertensive drug utilization in British Columbia, 2004-2019: a descriptive study
- PMID: 37527901
- PMCID: PMC10400081
- DOI: 10.9778/cmajo.20220023
Trends in antihypertensive drug utilization in British Columbia, 2004-2019: a descriptive study
Abstract
Background: Clinical guidelines for hypertension were updated with lower blood pressure targets following new studies in 2015; the real-world impact of these changes on antihypertensive drug use is unknown. We aimed to describe trends in antihypertensive drug utilization from 2004 to 2019 in British Columbia.
Methods: We conducted a longitudinal study to describe the annual prevalence and incidence rate of use of 5 antihypertensive drug classes (thiazides, angiotensin-converting enzyme [ACE] inhibitors, angiotensin II receptor blockers [ARBs], calcium channel blockers and β-blockers) among BC residents aged 30-75 years. We also conducted a cohort study to compare the risk of discontinuation and switch or add-on therapy between incident users of the above drug classes. We used linkable administrative health databases from BC. We performed a Fine-Gray competing risk analysis to estimate subhazard ratios.
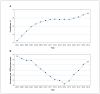
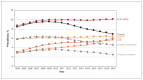
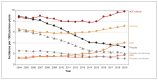
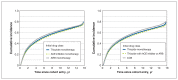
Results: Among BC residents aged 30-75 years (population: 2 376 282 [2004] to 3 014 273 [2019]), the incidence rate of antihypertensive drug use decreased from 23.7 per 1000 person-years in 2004 to 18.3 per 1000 person-years in 2014, and subsequently increased to 22.6 per 1000 person-years in 2019. The incidence rate of thiazide use decreased from 8.9 per 1000 person-years in 2004 to 3.2 per 1000 person-years in 2019, and incidence rates for the other drug classes increased. Incident users receiving thiazide monotherapy had an increased risk of discontinuing any antihypertensive treatment compared with ACE inhibitor monotherapy (subhazard ratio 0.96, 95% confidence interval [CI] 0.95-0.97), ARB monotherapy (subhazard ratio 0.84, 95% CI 0.81-0.87) and thiazide combination with ACE inhibitor or ARB (subhazard ratio 0.86, 95% CI 0.84-0.88), and had the highest risk of switching or adding on.
Interpretation: First-line use of thiazides continued to decrease despite a marked increase in incident antihypertensive therapy following updated guidelines; incident users receiving ARB monotherapy were least likely to discontinue, and incident users receiving thiazide monotherapy were more likely to switch or add on than users of other initial monotherapy or combination. Further research is needed on the factors influencing treatment decisions to understand the differences in trends and patterns of antihypertensive drug use.
© 2023 CMA Impact Inc. or its licensors.
Conflict of interest statement
Competing interests: None declared.
Figures

References
-
- Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephtrainnt of hypertension in adults and children. Can J Cardiol. 2020;36:596–624. - PubMed
-
- Lawes CMM, Vander Hoorn S, Rodgers A International Society of Hypertension. Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371:1513–8. - PubMed
-
- Padwal RS, Bienek A, McAlister FA, et al. Outcomes Research Task Force of the Canadian Hypertension Education Program. Epidemiology of hypertension in Canada: an update. Can J Cardiol. 2016;32:687–94. - PubMed
-
- Weaver CG, Clement FM, Campbell NRC, et al. Alberta Kidney Disease Network and the Interdisciplinary Chronic Disease Collaboration. Healthcare costs attributable to hypertension: Canadian population-based cohort study. Hypertension. 2015;66:502–8. - PubMed
-
- Daskalopoulou SS, Feldman RD, McAlister FA, et al. Hypertension Canada. The history of hypertension guidelines in Canada. Can J Cardiol. 2019;35:582–9. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
