Mitigation of Fibrosis after Myocardial Infarction in Rats by Using a Porcine Cholecyst Extracellular Matrix
- PMID: 37527924
- PMCID: PMC10702285
- DOI: 10.30802/AALAS-CM-22-000097
Mitigation of Fibrosis after Myocardial Infarction in Rats by Using a Porcine Cholecyst Extracellular Matrix
Abstract
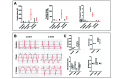
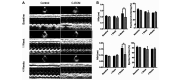
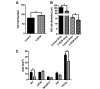
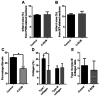
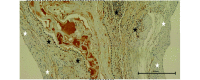
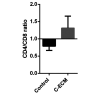
Fibrosis that occurs after nonfatal myocardial infarction (MI) is an irreversible reparative cardiac tissue remodeling process characterized by progressive deposition of highly cross-linked type I collagen. No currently available therapeutic strategy prevents or reverses MI-associated fibrotic scarring of myocardium. In this study, we used an epicardial graft prepared of porcine cholecystic extracellular matrix to treat experimental nonfatal MI in rats. Graft-assisted healing was characterized by reduced fibrosis, with scanty deposition of type I collagen. Histologically, the tissue response was associated with a favorable regenerative reaction predominated by CD4-positive helper T lymphocytes, enhanced angiogenesis, and infiltration of proliferating cells. These observations indicate that porcine cholecystic extracellular matrix delayed the fibrotic reaction and support its use as a potential biomaterial for mitigating fibrosis after MI. Delaying the progression of cardiac tissue remodeling may widen the therapeutic window for management of scarring after MI.
Figures








References
-
- Balakrishnan-Nair DK, Nair ND, Venugopal SK, Das VN, George S, Abraham MJ, Eassow S, Alison MR, Sainulabdeen A, Anilkumar TV. 2018. An immunopathological evaluation of the porcine cholecyst matrix as a muscle repair graft in a male rat abdominal wall defect model. Toxicol Pathol 46:169–183. 10.1177/0192623317752894. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

