Structural Changes of Cutaneous Immune Cells in Patients With Type 1 Diabetes and Their Relationship With Diabetic Polyneuropathy
- PMID: 37527931
- PMCID: PMC10393274
- DOI: 10.1212/NXI.0000000000200144
Structural Changes of Cutaneous Immune Cells in Patients With Type 1 Diabetes and Their Relationship With Diabetic Polyneuropathy
Erratum in
-
Missing Full Disclosures.Neurol Neuroimmunol Neuroinflamm. 2025 Jan;12(1):e200342. doi: 10.1212/NXI.0000000000200342. Epub 2024 Oct 30. Neurol Neuroimmunol Neuroinflamm. 2025. PMID: 39475708 Free PMC article. No abstract available.
Abstract
Background and objectives: Diabetic polyneuropathy (DPN) is a complication of diabetes characterized by pain or lack of peripheral sensation, but the underlying mechanisms are not yet fully understood. Recent evidence showed increased cutaneous macrophage infiltration in patients with type 2 diabetes and painful DPN, and this study aimed to understand whether the same applies to type 1 diabetes.
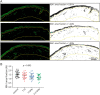
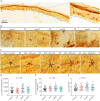
Methods: The study included 104 participants: 26 healthy controls and 78 participants with type 1 diabetes (participants without DPN [n = 24], participants with painless DPN [n = 29], and participants with painful DPN [n = 25]). Two immune cells, dermal IBA1+ macrophages and epidermal Langerhans cells (LCs, CD207+), were visualized and quantified using immunohistological labeling and stereological counting methods on skin biopsies from the participants. The IBA1+ macrophage infiltration, LC number density, LC soma cross-sectional area, and LC processes were measured in this study.
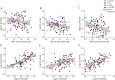
Results: Significant difference in IBA1+ macrophage expression was seen between the groups (p = 0.003), with lower expression of IBA1 in participants with DPN. No differences in LC morphologies (LC number density, soma cross-sectional area, and process level) were found between the groups (all p > 0.05). In addition, IBA1+ macrophages, but not LCs, correlated with intraepidermal nerve fiber density, Michigan neuropathy symptom inventory, (questionnaire and total score), severity of neuropathy as assessed by the Toronto clinical neuropathy score, and vibration detection threshold in the whole study cohort.
Discussion: This study showed expressional differences of cutaneous IBA1+ macrophages but not LC in participants with type 1 diabetes-induced DPN compared with those in controls. The study suggests that a reduction in macrophages may play a role in the development and progression of autoimmune-induced diabetic neuropathy.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Conflict of interest statement
P. Karlsson has received personal fees from Grünenthal, Alnylam, and Vertex Pharmaceuticals and has received a research grant from Merck outside the submitted work. Other authors have no conflicts of interest to state. Go to
Figures

References
-
- Gylfadottir SS, Christensen DH, Nicolaisen SK, et al. . Diabetic polyneuropathy and pain, prevalence, and patient characteristics: a cross-sectional questionnaire study of 5,514 patients with recently diagnosed type 2 diabetes. Pain. 2020;161(3):574-583. doi:10.1097/j.pain.0000000000001744 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
