Genetic inhibition of CARD9 accelerates the development of atherosclerosis in mice through CD36 dependent-defective autophagy
- PMID: 37528097
- PMCID: PMC10394049
- DOI: 10.1038/s41467-023-40216-x
Genetic inhibition of CARD9 accelerates the development of atherosclerosis in mice through CD36 dependent-defective autophagy
Abstract
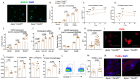
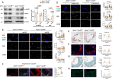
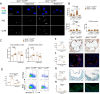
Caspase recruitment-domain containing protein 9 (CARD9) is a key signaling pathway in macrophages but its role in atherosclerosis is still poorly understood. Global deletion of Card9 in Apoe-/- mice as well as hematopoietic deletion in Ldlr-/- mice increases atherosclerosis. The acceleration of atherosclerosis is also observed in Apoe-/-Rag2-/-Card9-/- mice, ruling out a role for the adaptive immune system in the vascular phenotype of Card9 deficient mice. Card9 deficiency alters macrophage phenotype through CD36 overexpression with increased IL-1β production, increased lipid uptake, higher cell death susceptibility and defective autophagy. Rapamycin or metformin, two autophagy inducers, abolish intracellular lipid overload, restore macrophage survival and autophagy flux in vitro and finally abolish the pro-atherogenic effects of Card9 deficiency in vivo. Transcriptomic analysis of human CARD9-deficient monocytes confirms the pathogenic signature identified in murine models. In summary, CARD9 is a key protective pathway in atherosclerosis, modulating macrophage CD36-dependent inflammatory responses, lipid uptake and autophagy.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

