DNA hypermethylation driven by DNMT1 and DNMT3A favors tumor immune escape contributing to the aggressiveness of adrenocortical carcinoma
- PMID: 37528470
- PMCID: PMC10394822
- DOI: 10.1186/s13148-023-01534-5
DNA hypermethylation driven by DNMT1 and DNMT3A favors tumor immune escape contributing to the aggressiveness of adrenocortical carcinoma
Abstract
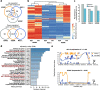
Background: Adrenocortical carcinoma is rare and aggressive endocrine cancer of the adrenal gland. Within adrenocortical carcinoma, a recently described subtype characterized by a CpG island methylator phenotype (CIMP) has been associated with an especially poor prognosis. However, the drivers of CIMP remain unknown. Furthermore, the functional relation between CIMP and poor clinical outcomes of patients with adrenocortical carcinoma stays elusive.
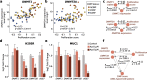
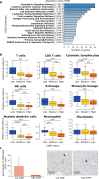
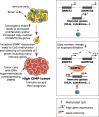
Results: Here, we show that CIMP in adrenocortical carcinoma is linked to the increased expression of DNA methyltransferases DNMT1 and DNMT3A driven by a gain of gene copy number and cell hyperproliferation. Importantly, we demonstrate that CIMP contributes to tumor aggressiveness by favoring tumor immune escape. This effect could be at least partially reversed by treatment with the demethylating agent 5-azacytidine.
Conclusions: In sum, our findings suggest that co-treatment with demethylating agents might enhance the efficacy of immunotherapy and could represent a novel therapeutic approach for patients with high CIMP adrenocortical carcinoma.
Keywords: 5-azacytidine; Adrenocortical carcinoma; CIMP; DNA methylation; DNA methyltransferases; DNMTs; Demethylating agents; Immune escape.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Abiven G, Coste J, Groussin L, Anract P, Tissier F, Legmann P, et al. Clinical and biological features in the prognosis of adrenocortical cancer: poor outcome of cortisol-secreting tumors in a series of 202 consecutive patients. J Clin Endocrinol Metab. 2006;91:2650–2655. doi: 10.1210/jc.2005-2730. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

