A multiscale predictive digital twin for neurocardiac modulation
- PMID: 37528537
- PMCID: PMC10528740
- DOI: 10.1113/JP284391
A multiscale predictive digital twin for neurocardiac modulation
Abstract
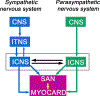
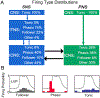
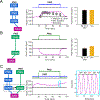
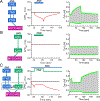
Cardiac function is tightly regulated by the autonomic nervous system (ANS). Activation of the sympathetic nervous system increases cardiac output by increasing heart rate and stroke volume, while parasympathetic nerve stimulation instantly slows heart rate. Importantly, imbalance in autonomic control of the heart has been implicated in the development of arrhythmias and heart failure. Understanding of the mechanisms and effects of autonomic stimulation is a major challenge because synapses in different regions of the heart result in multiple changes to heart function. For example, nerve synapses on the sinoatrial node (SAN) impact pacemaking, while synapses on contractile cells alter contraction and arrhythmia vulnerability. Here, we present a multiscale neurocardiac modelling and simulator tool that predicts the effect of efferent stimulation of the sympathetic and parasympathetic branches of the ANS on the cardiac SAN and ventricular myocardium. The model includes a layered representation of the ANS and reproduces firing properties measured experimentally. Model parameters are derived from experiments and atomistic simulations. The model is a first prototype of a digital twin that is applied to make predictions across all system scales, from subcellular signalling to pacemaker frequency to tissue level responses. We predict conditions under which autonomic imbalance induces proarrhythmia and can be modified to prevent or inhibit arrhythmia. In summary, the multiscale model constitutes a predictive digital twin framework to test and guide high-throughput prediction of novel neuromodulatory therapy. KEY POINTS: A multi-layered model representation of the autonomic nervous system that includes sympathetic and parasympathetic branches, each with sparse random intralayer connectivity, synaptic dynamics and conductance based integrate-and-fire neurons generates firing patterns in close agreement with experiment. A key feature of the neurocardiac computational model is the connection between the autonomic nervous system and both pacemaker and contractile cells, where modification to pacemaker frequency drives initiation of electrical signals in the contractile cells. We utilized atomic-scale molecular dynamics simulations to predict the association and dissociation rates of noradrenaline with the β-adrenergic receptor. Multiscale predictions demonstrate how autonomic imbalance may increase proclivity to arrhythmias or be used to terminate arrhythmias. The model serves as a first step towards a digital twin for predicting neuromodulation to prevent or reduce disease.
Keywords: arrhythmia; autonomic nervous system; cardiac electrophysiology; computational model; digital twins; parasympathetic; sympathetic nervous system.
© 2023 The Authors. The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Conflict of interest statement
Competing Interests
The authors declare no competing interests.
Figures





Comment in
-
The promise of cardiac neuromodulation: can computational modelling bridge the gap?J Physiol. 2023 Sep;601(17):3693-3694. doi: 10.1113/JP285309. Epub 2023 Aug 3. J Physiol. 2023. PMID: 37535053 Free PMC article. No abstract available.
Similar articles
-
Zebrafish heart as a model to study the integrative autonomic control of pacemaker function.Am J Physiol Heart Circ Physiol. 2016 Sep 1;311(3):H676-88. doi: 10.1152/ajpheart.00330.2016. Epub 2016 Jun 24. Am J Physiol Heart Circ Physiol. 2016. PMID: 27342878 Free PMC article.
-
HCN4 pacemaker channels attenuate the parasympathetic response and stabilize the spontaneous firing of the sinoatrial node.J Physiol. 2018 Mar 1;596(5):809-825. doi: 10.1113/JP275303. Epub 2018 Feb 6. J Physiol. 2018. PMID: 29315578 Free PMC article.
-
Autonomic control of the location and rate of the cardiac pacemaker in the sinoatrial fat pad of parasympathetically denervated dog hearts.J Cardiovasc Electrophysiol. 2002 Sep;13(9):896-901. doi: 10.1046/j.1540-8167.2002.00896.x. J Cardiovasc Electrophysiol. 2002. PMID: 12380928
-
Neuroscience in the heart: Recent advances in neurocardiac communication and its role in cardiac arrhythmias.Int J Biochem Cell Biol. 2020 May;122:105737. doi: 10.1016/j.biocel.2020.105737. Epub 2020 Mar 6. Int J Biochem Cell Biol. 2020. PMID: 32151724 Review.
-
Autonomic neuronal modulations in cardiac arrhythmias: Current concepts and emerging therapies.Physiol Behav. 2024 May 15;279:114527. doi: 10.1016/j.physbeh.2024.114527. Epub 2024 Mar 23. Physiol Behav. 2024. PMID: 38527577 Review.
Cited by
-
Definitions and Characteristics of Patient Digital Twins Being Developed for Clinical Use: Scoping Review.J Med Internet Res. 2024 Nov 13;26:e58504. doi: 10.2196/58504. J Med Internet Res. 2024. PMID: 39536311 Free PMC article.
-
Digital Twins of Biological Systems: A Narrative Review.IEEE Open J Eng Med Biol. 2024 Jul 12;5:670-677. doi: 10.1109/OJEMB.2024.3426916. eCollection 2024. IEEE Open J Eng Med Biol. 2024. PMID: 39184962 Free PMC article.
-
Medical Digital Twin: A Review on Technical Principles and Clinical Applications.J Clin Med. 2025 Jan 7;14(2):324. doi: 10.3390/jcm14020324. J Clin Med. 2025. PMID: 39860329 Free PMC article. Review.
-
Molecular simulations reveal intricate coupling between agonist-bound β-adrenergic receptors and G protein.iScience. 2025 Jan 2;28(2):111741. doi: 10.1016/j.isci.2024.111741. eCollection 2025 Feb 21. iScience. 2025. PMID: 39898043 Free PMC article.
-
Increased heart rate fragmentation in those with Williams-Beuren syndrome suggests nonautonomic mechanistic contributors to sudden death risk.Am J Physiol Heart Circ Physiol. 2024 Aug 1;327(2):H521-H532. doi: 10.1152/ajpheart.00601.2023. Epub 2024 Jun 21. Am J Physiol Heart Circ Physiol. 2024. PMID: 38904853 Free PMC article.
References
-
- Armour JA. (2004). Cardiac neuronal hierarchy in health and disease. Am J Physiol Regul Integr Comp Physiol 287, R262–271. - PubMed
-
- Barducci A, Bussi G & Parrinello M. (2008). Well-Tempered Metadynamics: A Smoothly Converging and Tunable Free-Energy Method. Physical Review Letters 100, 020603. - PubMed

