Sustained oral spermidine supplementation rescues functional and structural defects in COL6-deficient myopathic mice
- PMID: 37528588
- PMCID: PMC10621270
- DOI: 10.1080/15548627.2023.2241125
Sustained oral spermidine supplementation rescues functional and structural defects in COL6-deficient myopathic mice
Abstract
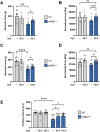
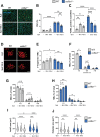
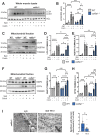
COL6 (collagen type VI)-related myopathies (COL6-RM) are a distinct group of inherited muscle disorders caused by mutations of COL6 genes and characterized by early-onset muscle weakness, for which no cure is available yet. Key pathophysiological features of COL6-deficient muscles involve impaired macroautophagy/autophagy, mitochondrial dysfunction, neuromuscular junction fragmentation and myofiber apoptosis. Targeting autophagy by dietary means elicited beneficial effects in both col6a1 null (col6a1-/-) mice and COL6-RM patients. We previously demonstrated that one-month per os administration of the nutraceutical spermidine reactivates autophagy and ameliorates myofiber defects in col6a1-/- mice but does not elicit functional improvement. Here we show that a 100-day-long spermidine regimen is able to rescue muscle strength in col6a1-/- mice, with also a beneficial impact on mitochondria and neuromuscular junction integrity, without any noticeable side effects. Altogether, these data provide a rationale for the application of spermidine in prospective clinical trials for COL6-RM.Abbreviations: AChR: acetylcholine receptor; BTX: bungarotoxin; CNF: centrally nucleated fibers; Colch: colchicine; COL6: collagen type VI; COL6-RM: COL6-related myopathies; MAP1LC3/LC3: microtubule-associated protein 1 light chain 3; NMJ: neuromuscular junction; Spd: spermidine; SQSTM1/p62: sequestosome 1; TA: tibialis anterior; TOMM20: translocase of outer mitochondrial membrane 20; TUNEL: terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling.
Keywords: Autophagy; collagen VI; nutraceutical; skeletal muscle; spermidine.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
