The Lucerne Toolbox 2 to optimise axillary management for early breast cancer: a multidisciplinary expert consensus
- PMID: 37528842
- PMCID: PMC10388578
- DOI: 10.1016/j.eclinm.2023.102085
The Lucerne Toolbox 2 to optimise axillary management for early breast cancer: a multidisciplinary expert consensus
Abstract
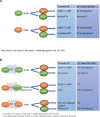
Clinical axillary lymph node management in early breast cancer has evolved from being merely an aspect of surgical management and now includes the entire multidisciplinary team. The second edition of the "Lucerne Toolbox", a multidisciplinary consortium of European cancer societies and patient representatives, addresses the challenges of clinical axillary lymph node management, from diagnosis to local therapy of the axilla. Five working packages were developed, following the patients' journey and addressing specific clinical scenarios. Panellists voted on 72 statements, reaching consensus (agreement of 75% or more) in 52.8%, majority (51%-74% agreement) in 43.1%, and no decision in 4.2%. Based on the votes, targeted imaging and standardized pathology of lymph nodes should be a prerequisite to planning local and systemic therapy, axillary lymph node dissection can be replaced by sentinel lymph node biopsy ( ± targeted approaches) in a majority of scenarios; and positive patient outcomes should be driven by both low recurrence risks and low rates of lymphoedema.
Keywords: Axillary management; Breast cancer; Consensus; Patient journey.
© 2023 The Author(s).
Conflict of interest statement
Ana Bosch: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Lilly, Roche, Novartis; Support for attending meetings and/or travel from Roche; Participation on a Data Safety Monitoring Board or Advisory; Giuseppe Curigliano: Consulting fees from BMS, Roche, Pfizer, Novartis, Lilly, Astra Zeneca, Daichii Sankyo, Merck, Seagen, Ellipsis, Gilead; Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Lilly, Pfizer, Relay; Support for attending meetings and/or travel from Daichii Sankyo; Jana De Boniface: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Astra Zeneca; Carsten Denkert: Grants from Myriad, Roche, BMBF, European Commission, GBG Foundation; consulting fees from MSD Oncology, Daiichi Sankyo, Molecular Health, AstraZeneca, Roche, Lilly; Peter Dubsky: Honoraria for presentations and Advisory from Roche and Astra Zeneca to Hirslanden Klinik St. Anna; Michael Knauer: Grants or contracts Agendia BV; Consulting fees from Myriad, Exact Sciences; Thorsten Kühn: Consulting fees from Merit Medical, Endomag, Hologic, Sirius Medical; Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from GILEAD, MSD, Pfizer, Exact Sciences; Support for attending meetings and/or travel from Gilead, MSD; Sibylle Loibl: Grants or contracts from Abbvie, AZ, BMS/Celgene, DAichi Synkyo, Gilead, Molecular Health, Novartis, Pfizer, Roche; Royalties or licenses for Ki 67 Analyser, VM SCOPE; Consulting fees from AZ, Amgen, Abbvie, BMS, Celgene, Daichi Sankyo Eirgenix, Gilead, Lilly, Merck, MSD, Novartis, Olema, Pfizer, Pierre Fabre, Relay Therapeutics, Incyte, Roche, Sanofi, Seagen; Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AZ, Gilead, Roche, Seagen, MSD, Pierre Fabre, MEDSCAPE; Patents planned, issued or pending EP14153692.0; EP21152186.9; EP15702464.7; EP15702464.7; Participation on a Data; Safety Monitoring Board or Advisory Board from Daichi Sankyo; Receipt of equipment, materials, drugs, medical writing, gifts or other services Daichiy Sankyo, Gilead, Novartis, AstraZeneca, Roche, Seagen; Icro Meattini: Consulting fees from Novartis, Pfizer, Eli Lilly, Seagen, Gilead; Katja Pinker: Consulting fees from Genentech, Inc., Merantix Healthcare, AURA Health Technologies GmbH; Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: European Society of Breast Imaging (active), Bayer (active), Siemens Healthineers (ended), DKD 2019 (ended), Olea Medical (ended), Roche (ended); Trine Tramm: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Pfizer, Roche; Support for attending meetings and/or travel from Roche and Novartis; Jong Han Yu: Consulting fees from Gencurix; Walter Paul Weber: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from MSD; all other others have no conflicts of interest to declare.
Figures

References
-
- Dubsky P., Pinker K., Cardoso F., et al. Breast conservation and axillary management after primary systemic therapy in patients with early-stage breast cancer: the Lucerne toolbox. Lancet Oncol. 2021;22:e18–e28. - PubMed
-
- Fisher B., Anderson S.J. The breast cancer alternative hypothesis: is there evidence to justify replacing it? J Clin Oncol. 2010;28:366–374. - PubMed
-
- Senkus E., Cardoso M.J., Kaidar-Person O., Łacko A., Meattini I., Poortmans P. De-escalation of axillary irradiation for early breast cancer – has the time come? Cancer Treat Rev. 2021;101 - PubMed
-
- de Wild S.R., de Munck L., Simons J.M., et al. De-escalation of radiotherapy after primary chemotherapy in cT1–2N1 breast cancer (RAPCHEM; BOOG 2010–03): 5-year follow-up results of a Dutch, prospective, registry study. Lancet Oncol. 2022;23:1201–1210. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

