Optimizing and developing a scalable, chemically defined, animal component-free lentiviral vector production process in a fixed-bed bioreactor
- PMID: 37528866
- PMCID: PMC10388200
- DOI: 10.1016/j.omtm.2023.06.011
Optimizing and developing a scalable, chemically defined, animal component-free lentiviral vector production process in a fixed-bed bioreactor
Abstract
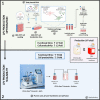
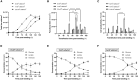
Lentiviral vectors (LVVs) play a critical role in gene delivery for ex vivo gene-modified cell therapies. However, the lack of scalable LVV production methods and the high cost associated with them may limit their use. In this work, we demonstrate the optimization and development of a scalable, chemically defined, animal component-free LVV production process using adherent human embryonic kidney 293T cells in a fixed-bed bioreactor. The initial studies focused on the optimization of the culture process in 2D static cultures. Process changes such as decreasing cell seeding density on day 0 from 2.5 × 104 to 5 × 103 cells/cm2, delaying the transient transfection from 24 to 120 h post-seeding, reducing plasmid DNA to 167 ng/cm2, and adding 5 mM sodium butyrate 6 h post-transfection improved functional LVV titers by 26.9-fold. The optimized animal component-free production process was then transferred to the iCELLis Nano bioreactor, a fixed-bed bioreactor, where titers of 1.2 × 106 TU/cm2 were achieved when it was operated in perfusion. In this work, comparable functional LVV titers were obtained with FreeStyle 293 Expression medium and the conventional Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum both at small and large scale.
Keywords: animal component-free; chemically defined; fixed-bed bioreactor; gene therapy; iCELLis Nano bioreactor; immunotherapy; lentiviral vectors; scalability; serum-free; virus.
© 2023 The Authors.
Conflict of interest statement
The work was funded, in part, by the UCL-Pall Biotech Center of Excellence as noted in the acknowledgments. It should be noted that M.-LC. and J.W. are employees of Pall Corporation at the time of submission.
Figures





References
-
- U.S. Food and Drug Administration KYMRIAH® (tisagenlecleucel) [package insert] 2017. https://www.fda.gov/media/107296/download
-
- U.S. Food and Drug Administration BREYANZI® (lisocabtagene maraleucel) [package insert] 2021. https://www.fda.gov/media/145711/download
-
- U.S. Food and Drug Administration ABECMA® (idecabtagene vicleucel) [package insert] 2021. https://www.fda.gov/media/147055/download
-
- U.S. Food and Drug Administration CARVYKTI™ (ciltacabtagene autoleucel) [package insert] 2022. https://www.fda.gov/media/156560/download
LinkOut - more resources
Full Text Sources

