Human precision-cut cystic duct and gallbladder slices: a novel method for studying cholangiopathies
- PMID: 37528870
- PMCID: PMC10387522
- DOI: 10.3389/fped.2023.1058319
Human precision-cut cystic duct and gallbladder slices: a novel method for studying cholangiopathies
Abstract
Background and aims: Precision-cut tissue slices (PCTS) are widely used as an ex vivo culture tissue culture technique to study pathogeneses of diseases and drug activities in organs in vitro. A novel application of the PCTS model may be in the field of translational research into cholangiopathies such as biliary atresia. Therefore, the aim of this study was to apply the precision-cut slice technique to human bile duct and gallbladder tissue.
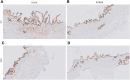
Methods: Cystic duct and gallbladder tissue derived from patients undergoing a cholecystectomy were collected, preserved and used for preparation of precision-cut cystic duct slices (PCCDS) and precision-cut gallbladder slices (PCGS). The PCCDS and PCGS were prepared using a mechanical tissue slicer and subsequently incubated for 24 and 48 h respectively in William's Medium E (WME) culture medium. Viability was assessed based on ATP/protein content and tissue morphology [hematoxylin and eosin (H&E) staining].
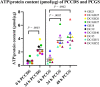
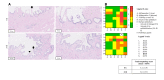
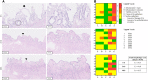
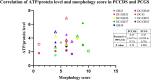
Results: It was shown that viability, assessed by the ATP/protein content and morphology, of the PCCDS (n = 8) and PCGS (n = 8) could be maintained over the 24 and 48 h incubation period respectively. ATP/protein content of the PCCDS increased significantly from 0.58 ± 0.13 pmol/µg at 0 h to 2.4 ± 0.29 pmol/µg after 24 h incubation (P = .0003). A similar significant increase from 0.94 ± 0.22 pmol/µg at 0 h to 3.7 ± 0.41 pmol/µg after 24 h (P = .0005) and 4.2 ± 0.47 pmol/µg after 48 h (P = .0002) was observed in the PCGS. Morphological assessment of the PCCDS and PCGS showed viable tissue at 0 h and after 24 and 48 h incubation respectively.
Conclusion: This study is the first to report on the use of the PCTS model for human gallbladder and cystic duct tissue. PCCDS and PCGS remain viable for an incubation period of at least 24 h, which makes them suitable for research purposes in the field of cholangiopathies, including biliary atresia.
Keywords: cholangiopathies; cystic duct; ex vivo culture technique; gallbladder; precision-cut tissue slices (PCTS).
© 2023 Fridrichs, Hamel, Kelder, van den Hoed, van den Heuvel, Hulscher and Olinga.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

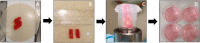

References
LinkOut - more resources
Full Text Sources

