Recognition of H2AK119ub plays an important role in RSF1-regulated early Xenopus development
- PMID: 37529237
- PMCID: PMC10389277
- DOI: 10.3389/fcell.2023.1168643
Recognition of H2AK119ub plays an important role in RSF1-regulated early Xenopus development
Abstract
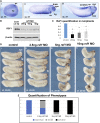
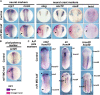
Polycomb group (PcG) proteins are key regulators of gene expression and developmental programs via covalent modification of histones, but the factors that interpret histone modification marks to regulate embryogenesis are less studied. We previously identified Remodeling and Spacing Factor 1 (RSF1) as a reader of histone H2A lysine 119 ubiquitination (H2AK119ub), the histone mark deposited by Polycomb Repressive Complex 1 (PRC1). In the current study, we used Xenopus laevis as a model to investigate how RSF1 affects early embryonic development and whether recognition of H2AK119ub is important for the function of RSF1. We showed that knockdown of Xenopus RSF1, rsf1, not only induced gastrulation defects as reported previously, but specific targeted knockdown in prospective neural precursors induced neural and neural crest defects, with reductions of marker genes. In addition, similar to knockdown of PRC1 components in Xenopus, the anterior-posterior neural patterning was affected in rsf1 knockdown embryos. Binding of H2AK119ub appeared to be crucial for rsf1 function, as a construct with deletion of the UAB domain, which is required for RSF1 to recognize the H2AK119ub nucleosomes, failed to rescue rsf1 morphant embryos and was less effective in interfering with early Xenopus development when ectopically expressed. Furthermore, ectopic deposition of H2AK119ub on the Smad2 target gene gsc using a ring1a-smad2 fusion protein led to ectopic recruitment of RSF1. The fusion protein was inefficient in inducing mesodermal markers in the animal region or a secondary axis when expressed in the ventral tissues. Taken together, our results reveal that rsf1 modulates similar developmental processes in early Xenopus embryos as components of PRC1 do, and that RSF1 acts at least partially through binding to the H2AK119ub mark via the UAB domain during development.
Keywords: H2AK119ub; PRC1; RSF1; UAB domain; Xenopus laevis; development; mesoderm; neural and neural crest.
Copyright © 2023 Parast, Yu, Chen, Dickinson, Chang and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Role of remodeling and spacing factor 1 in histone H2A ubiquitination-mediated gene silencing.Proc Natl Acad Sci U S A. 2017 Sep 19;114(38):E7949-E7958. doi: 10.1073/pnas.1711158114. Epub 2017 Aug 30. Proc Natl Acad Sci U S A. 2017. PMID: 28855339 Free PMC article.
-
Read-write mechanisms of H2A ubiquitination by Polycomb repressive complex 1.Nature. 2024 Dec;636(8043):755-761. doi: 10.1038/s41586-024-08183-5. Epub 2024 Nov 13. Nature. 2024. PMID: 39537923
-
Histone H2A deubiquitinases in the transcriptional programs of development and hematopoiesis: a consolidated analysis.Int J Biochem Cell Biol. 2023 Apr;157:106384. doi: 10.1016/j.biocel.2023.106384. Epub 2023 Feb 2. Int J Biochem Cell Biol. 2023. PMID: 36738766
-
Histone Mono-Ubiquitination in Transcriptional Regulation and Its Mark on Life: Emerging Roles in Tissue Development and Disease.Cells. 2022 Aug 4;11(15):2404. doi: 10.3390/cells11152404. Cells. 2022. PMID: 35954248 Free PMC article. Review.
-
Biological functions of chromobox (CBX) proteins in stem cell self-renewal, lineage-commitment, cancer and development.Bone. 2021 Feb;143:115659. doi: 10.1016/j.bone.2020.115659. Epub 2020 Sep 24. Bone. 2021. PMID: 32979540 Review.
Cited by
-
Dyrk1a is required for craniofacial development in Xenopus laevis.Dev Biol. 2024 Jul;511:63-75. doi: 10.1016/j.ydbio.2024.04.004. Epub 2024 Apr 15. Dev Biol. 2024. PMID: 38621649 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

