Real-world data in patients with congenital hemophilia and inhibitors: final data from the FEIBA Global Outcome (FEIBA GO) study
- PMID: 37529276
- PMCID: PMC10387704
- DOI: 10.1177/20406207231184323
Real-world data in patients with congenital hemophilia and inhibitors: final data from the FEIBA Global Outcome (FEIBA GO) study
Abstract
Background: The bypassing agent, activated prothrombin complex concentrate [aPCC, FEIBA (factor VIII inhibitor bypass activity); Baxalta US Inc, a Takeda company, Lexington, MA, USA], is indicated for the treatment of bleeding episodes, perioperative management, and routine prophylaxis in patients with hemophilia A or B with inhibitors. In certain countries, aPCC is also indicated for the treatment of bleeding episodes and perioperative management in patients with acquired hemophilia A.
Objectives: To describe long-term, real-world effectiveness, safety, and quality-of-life outcomes for patients with congenital hemophilia A or B and high-responding inhibitors receiving aPCC treatment in routine clinical practice.
Design: FEIBA Global Outcome (FEIBA GO; EUPAS6691) was a prospective, observational study.
Methods: Investigators determined the treatment regimen and clinical monitoring frequency. The planned patient observation period was 4 years. Data are from the safety analysis set (patients who received ⩾1 aPCC infusion).
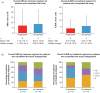
Results: Overall, 50 patients received either aPCC prophylaxis (n = 37) or on-demand therapy (n = 13) at screening [hemophilia A, n = 49; hemophilia B, n = 1; median (range) age, 16.5 [2-71] years). Mean ± standard deviation overall annualized bleeding rate and annualized joint bleeding rate for patients receiving prophylaxis were 6.82 ± 11.52 and 3.77 ± 5.71, respectively, and for patients receiving on-demand therapy were 10.94 ± 11.27 and 6.94 ± 7.39, respectively. Overall, 177 and 31 adverse events (AEs) were reported in 28 of 40 and 10 of 13 patients receiving prophylaxis or on-demand therapy, respectively. Two serious AEs were considered possibly related to aPCC: acute myocardial infarction due to coronary artery embolism in one patient receiving prophylaxis. No thrombotic microangiopathy was reported. No AEs resulted in death.
Conclusion: This study demonstrated the long-term, real-world effectiveness and consistent safety profile of aPCC as on-demand therapy and prophylactic treatment in patients with hemophilia and high-responding inhibitors.
Trial registry: FEIBA Global Outcome Study; EUPAS6691 https://www.encepp.eu/encepp/viewResource.htm?id=32774.
Keywords: factor VIII inhibitor bypassing activity; hemophilia A; hemophilia B; inhibitors; observational study.
© The Author(s), 2023.
Conflict of interest statement
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Carmen Escuriola Ettingshausen: grant/research support [Biotest, CSL Behring, Octapharma, Shire (a Takeda company), Sobi]; honoraria for lectures/advisory boards [Bayer, Biotest, CSL Behring, Grifols, Kedrion, LFB, Novo Nordisk, Octapharma, Pfizer, Roche/Chugai, Shire (a Takeda company), Sobi]; and consultancy [BioMarin, Biotest, CSL Behring, Grifols, Novo Nordisk, Octapharma, Roche/Chugai, Shire (a Takeda company), Sobi]. Cedric Hermans: grant/research support [Bayer, Pfizer, Shire (a Takeda company), Sobi]; honoraria for lectures/advisory boards [Bayer, Biogen, CAF-DCF, CSL Behring, Kedrion, LFB, Novo Nordisk, Octapharma, Pfizer, Roche, Shire (a Takeda company), Sobi]; and consultancy [Bayer, Biogen, CAF-DCF, CSL Behring, LFB, Novo Nordisk, Octapharma, Pfizer, Roche, Shire (a Takeda company), Sobi]. Pål A. Holme: grant/research support [Bayer, Octapharma, Pfizer, Shire (a Takeda company), Sobi]; and honoraria for lectures/advisory boards [Bayer, CSL Behring, Novo Nordisk, Pfizer, Shire (a Takeda company), Sobi]. Ana R. Cid: honoraria for lectures/advisory boards [Novo Nordisk, Roche, Shire (a Takeda company), Sobi]; and consultancy (Roche, Sobi). Kate Khair: grant/research support [Baxalta/Shire (a Takeda company), CSL Behring, Novo Nordisk, Pfizer, Roche, Sobi, uniQure]; honoraria for lectures/advisory boards [Bayer, Novo Nordisk, Pfizer, Roche, Shire (a Takeda company), Sobi]; consultancy [Bayer, Novo Nordisk, Roche, Shire (a Takeda company), Sobi]; support attending meetings and/or travel (Bayer, Novo Nordisk); leadership or fiduciary role in other board, society, committee or advocacy group (Vice President board of Trustees The Haemophilia Society); and stock or stock options (Haemnet Ltd, Medikhair). Johannes Oldenburg: grant/research support [Bayer, Biotest, CSL Behring, Novo Nordisk, Octapharma, Pfizer, Shire (a Takeda company)]; honoraria for lectures/advisory boards [Bayer, Biogen, Biotest, Chugai, CSL Behring, Grifols, Novo Nordisk, Octapharma, Pfizer, Roche, Shire (a Takeda company), Sobi]; and consultancy [Bayer, Biogen, Biotest, Chugai, CSL Behring, Grifols, Novo Nordisk, Octapharma, Pfizer, Roche, Shire (a Takeda company), Sobi]. Claude Négrier: grant/research support [CSL Behring, Octapharma, Shire (a Takeda company), Sobi]; and consultancy [Bayer, BioMarin, CSL Behring, Freeline, LFB, Novo Nordisk, Octapharma, Pfizer, Roche, Sanofi, Shire (a Takeda company), Sobi, Spark]. Jaco Botha: employee of Takeda Pharmaceuticals International AG, and Takeda stock owner. Aurelia Lelli: employee of Takeda Pharmaceuticals International AG, and Takeda stock owner. Jerzy Windyga: grant/research support and honoraria for lectures [Amgen, Alnylam, Baxalta/Shire (a Takeda company), Bayer, CSL Behring, LFB, Novartis, Novo Nordisk, Octapharma, Pfizer, Roche, Sanofi, Siemens, Sobi, Swixx Biopharma].
Figures



References
-
- Srivastava A, Santagostino E, Dougall A, et al.. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia 2020; 26(Suppl. 6): 1–158. - PubMed
-
- Gouw SC, Van den Berg HM, Fischer K, et al.. Intensity of factor VIII treatment and inhibitor development in children with severe hemophilia A: the RODIN study. Blood 2013; 121: 4046–4055. - PubMed
-
- Van den Berg HM, Fischer K, Carcao M, et al.. Timing of inhibitor development in more than 1000 previously untreated patients with severe hemophilia A. Blood 2019; 134: 317–320 - PubMed
LinkOut - more resources
Full Text Sources

