Performance evaluation of flexible macrocycle docking in AutoDock
- PMID: 37529284
- PMCID: PMC10392634
- DOI: 10.1017/qrd.2022.18
Performance evaluation of flexible macrocycle docking in AutoDock
Abstract
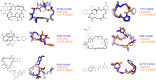
Macrocycles represent an important class of ligands, both in natural products and designed drugs. In drug design, macrocyclizations can impart specific ligand conformations and contribute to passive permeation by encouraging intramolecular H-bonds. AutoDock-GPU and Vina can model macrocyclic ligands flexibly, without requiring the enumeration of macrocyclic conformers before docking. Here, we characterize the performance of the method for handling macrocyclic compounds, which is implemented and the default behaviour for ligand preparation with our ligand preparation pipeline, Meeko. A pseudoatom is used to encode bond geometry and produce an anisotropic closure force for macrocyclic rings. This method is evaluated on a diverse set of small molecule and peptide macrocycles, ranging from 7- to 33-membered rings, showing little accuracy loss compared to rigid redocking of the X-ray macrocycle conformers. This suggests that for conformationally flexible macrocycles with unknown binding modes, this method can be effectively used to predict the macrocycle conformation.
Keywords: autodock; docking; macrocycles.
© The Author(s) 2022.
Figures










References
-
- Arnison PG, Bibb MJ, Bierbaum G, Bowers AA, Bugni TS, Bulaj G, Camarero JA, Campopiano DJ, Challis GL, Clardy J, Cotter PD, Craik DJ, Dawson M, Dittmann E, Donadio S, Dorrestein PC, Entian KD, Fischbach MA, Garavelli JS, Göransson U, Gruber CW, Haft DH, Hemscheidt TK, Hertweck C, Hill C, Horswill AR, Jaspars M, Kelly WL, Klinman JP, Kuipers OP, Link AJ, Liu W, Marahiel MA, Mitchell DA, Moll GN, Moore BS, Müller R, Nair SK, Nes IF, Norris GE, Olivera BM, Onaka H, Patchett ML, Piel J, Reaney MJ, Rebuffat S, Ross RP, Sahl HG, Schmidt EW, Selsted ME, Severinov K, Shen B, Sivonen K, Smith L, Stein T, Süssmuth RD, Tagg JR, Tang GL, Truman AW, Vederas JC, Walsh CT, Walton JD, Wenzel SC, Willey JM and van der Donk WA (2013) Ribosomally synthesized and post-translationally modified peptide natural products: Overview and recommendations for a universal nomenclature. Natural Product Reports 30(1), 108–160. 10.1039/c2np20085f - DOI - PMC - PubMed
-
- Case DA, Aktulga HM, Belfon K, Ben-Shalom IY, Berryman JT, Brozell SR, Cerutti DS, Cheatham TE, Cisneros GA, Cruzeiro VWD, Darden TA, Duke RE, Giambasu G, Gilson MK, Gohlke H, Goetz AW, Harris R, Izadi S, Izmailov SA, Kasavajhala K, Kaymak MC, King E, Kovalenko A, Kurtzman T, Lee TS, LeGrand S, Li P, Lin C, Liu J, Luchko T, Luo R, Machado M, Man V, Manathunga M, Merz KM, Miao Y, Mikhailovskii O, Monard G, Nguyen H, O’Hearn KA, Onufriev A, Pan F, Pantano S, Qi R, Rahnamoun A, Roe DR, Roitberg A, Sagui C, Schott-Verdugo S, Shajan A, Shen J, Simmerling CL, Skrynnikov NR, Smith J, Swails J, Walker RC, Wang J, Wang J, Wei H, Wolf RM, Wu X, Xiong Y, Xue Y, York DM, Zhao S and Kollman PA (2022) Amber 2022, University of California, San Francisco.
-
- Bottchscore (n.d.). Available at https://github.com/forlilab/bottchscore (accessed May 2022).
LinkOut - more resources
Full Text Sources
