Membrane estrogen receptor-α contributes to female protection against high-fat diet-induced metabolic disorders
- PMID: 37529599
- PMCID: PMC10390233
- DOI: 10.3389/fendo.2023.1215947
Membrane estrogen receptor-α contributes to female protection against high-fat diet-induced metabolic disorders
Abstract
Background: Estrogen Receptor α (ERα) is a significant modulator of energy balance and lipid/glucose metabolisms. Beyond the classical nuclear actions of the receptor, rapid activation of intracellular signaling pathways is mediated by a sub-fraction of ERα localized to the plasma membrane, known as Membrane Initiated Steroid Signaling (MISS). However, whether membrane ERα is involved in the protective metabolic actions of endogenous estrogens in conditions of nutritional challenge, and thus contributes to sex differences in the susceptibility to metabolic diseases, remains to be clarified.
Methods: Male and female C451A-ERα mice, harboring a point mutation which results in the abolition of membrane localization and MISS-related effects of the receptor, and their wild-type littermates (WT-ERα) were maintained on a normal chow diet (NCD) or fed a high-fat diet (HFD). Body weight gain, body composition and glucose tolerance were monitored. Insulin sensitivity and energy balance regulation were further investigated in HFD-fed female mice.
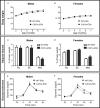
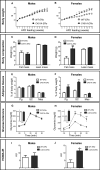
Results: C451A-ERα genotype had no influence on body weight gain, adipose tissue accumulation and glucose tolerance in NCD-fed mice of both sexes followed up to 7 months of age, nor male mice fed a HFD for 12 weeks. In contrast, compared to WT-ERα littermates, HFD-fed C451A-ERα female mice exhibited: 1) accelerated fat mass accumulation, liver steatosis and impaired glucose tolerance; 2) whole-body insulin resistance, assessed by hyperinsulinemic-euglycemic clamps, and altered insulin-induced signaling in skeletal muscle and liver; 3) significant decrease in energy expenditure associated with histological and functional abnormalities of brown adipose tissue and a defect in thermogenesis regulation in response to cold exposure.
Conclusion: Besides the well-characterized role of ERα nuclear actions, membrane-initiated ERα extra-nuclear signaling contributes to female, but not to male, protection against HFD-induced obesity and associated metabolic disorders in mouse.
Keywords: estrogen receptor alpha (ERα); insulin resistance; membrane-initiated steroid signaling; obesity; sex differences; thermogenesis.
Copyright © 2023 Fabre, Tramunt, Montagner, Mouly, Riant, Calmy, Adlanmerini, Fontaine, Burcelin, Lenfant, Arnal and Gourdy.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

