Insight into electrochemical degradation of Cartap (in Padan 95SP) by boron-doped diamond electrode: kinetic and effect of water matrices
- PMID: 37529726
- PMCID: PMC10390180
- DOI: 10.55730/1300-0527.3476
Insight into electrochemical degradation of Cartap (in Padan 95SP) by boron-doped diamond electrode: kinetic and effect of water matrices
Abstract
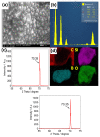
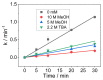
In this work, the kinetic electrochemical degradation of Cartap (CT) (in Padan 95 SP) at boron-doped diamond (BDD) electrode was investigated. This study indicated that the degradation of CT underwent both direct and indirect oxidations. Water matrices can either accelerate or inhibit the removal efficiency of CT: adding 15 mM Cl- improved kCT from 0.039 min-1 to 0.054 min-1 (increased by 38%), while kCT decreased by 61.5% and 64% when increasing the concentration of HCO3- and humic acid (HA) to 15 mM and 15 mg L-1, respectively. CT degradation was inhibited in the presence of methanol (MeOH) and tert-butanol (TBA) due to the scavenging effect of those chemicals toward reactive species. The contribution of reactive oxidants was calculated as: DET (direct electron transfer) accounted for 15%; •OH accounted for 61.5%; SO4•- accounted for 12.8%; ROS (the other reactive oxygen species) accounted for 8.5%. The transformation pathways of major reactive species were established.
Keywords: BDD electrode; Cartap degradation; cyclic voltammetry; kinetic; linear sweep voltammetry; mechanism.
© TÜBİTAK.
Conflict of interest statement
Conflict of interest The author declare no competing financial interest.
Figures





References
-
- Standards BT. Determination of cartap hydrochloride content (spectrophotometric method) copyrighted by Bureau of Indian Standards. IS. 1994;14159
-
- Costa TD, Santos J, Silva D, Martinez-Huitle C. BDD-Electrolysis of Oxalic Acid in Diluted Acidic Solutions. Journal of the Brazilian Chemical Society. 2019;30(7):1541–1547. doi: 10.21577/0103-5053.20190051. - DOI
-
- Dávila OO, Bergeron LL, Gutiérrez PR, Jiménez MMD, Sirés I, Brillas E, Navarro AFR, Arandes JB, Llopis JVS. Electrochemical oxidation of dibenzothiophene compounds on BDD electrode in acetonitrile-water medium. Journal of Electroanalytical Chemistry. 2019;847:113172. doi: 10.1016/j.jelechem.2019.05.054. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
