Confirmatory Factor Analysis and Measurement Invariance of the Functional Assessment of Cancer Therapy Lung Cancer Utility Index (FACT-LUI)
- PMID: 37529767
- PMCID: PMC10388633
- DOI: 10.1177/23814683231186992
Confirmatory Factor Analysis and Measurement Invariance of the Functional Assessment of Cancer Therapy Lung Cancer Utility Index (FACT-LUI)
Abstract
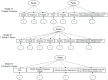
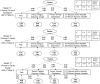
Background. A portion of the Functional Assessment of Cancer Therapy-Lung (FACT-L) instrument contributed to a previously published utility index, the FACT Lung Utility Index or FACT-LUI. Six FACT items representing lung cancer quality of life covered fatigue, pain, dyspnea, cough, anxiety, and depression. Two FACT items had been previously combined by the index authors into one for nausea and/or appetite loss, resulting in 7 final domains. Methods. The objective was to perform measurement invariance testing within a confirmatory factor analysis (CFA) framework to support the feasibility of using the FACT-LUI for non-preference-based psychometric applications. The original index patients comprised group 1, and similar FACT patient data (n = 249) from another published study comprised group 2. One 2-factor model and two 1-factor CFA models were evaluated to assess measurement invariance across groups, using varying degrees of item parceling and a small number of residual covariances, all justified by the literature. Results. The 1-factor models were most optimal. A 1-factor model with 1 pair of items parceled showed invariance to the partial scalar level using usual fit criteria across groups, requiring 2 unconstrained intercepts. A 1-factor model with 3 pairs of justified parcels showed full configural, metric, and scalar invariance across groups. Conclusions. The FACT-LUI items fit a partially to fully invariant 1-factor model, suggesting feasibility for non-preference-based applications. Implications. Results suggest useful incorporation of the FACT-LUI into clinical trials with no substantial increased respondent burden, allowing preference-based and other psychometric applications from the same index items.
Highlights: This work suggests that in addition to being originally designed for use as a utility index, the 7 FACT-LUI items together also fit simple CFA and measurement invariance models. This less expected result indicates that these items as a group are also potentially useful in non-preference-based applications.Clinical trials can make for challenging decisions concerning which patient-reported outcome measures to include without being burdensome. However, the literature suggests a need for improved reporting of quality of life in lung cancer in particular as well as cancer in general. Inclusion of more disease-specific items such as the FACT-LUI may allow for information gathering of both preference-based and non-preference-based data with less demand on patients, similar to what has been done with some generic instruments.
Keywords: factor analysis; health-related quality of life measurement; preference-based measurement; psychometrics; structural equation modeling; utility index.
© The Author(s) 2023.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: American Cancer Society (#126904-PEP-14-206-01-PCSM). Financial support for this study was provided entirely by a grant from the American Cancer Society. The funding arrangement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report.
Figures


References
-
- Kuon J, Blasi M, Unsold L, et al.. Impact of molecular alterations on quality of life and prognostic understanding over time in patients with incurable lung cancer: a multicenter, longitudinal, prospective cohort study. Support Care Cancer. 2022;30(4):3131–40. doi:10.1007/s00520-021-06736-2 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

