miR-122-5p is involved in posttranscriptional regulation of the mitochondrial thiamin pyrophosphate transporter (SLC25A19) in pancreatic acinar cells
- PMID: 37529835
- PMCID: PMC10642993
- DOI: 10.1152/ajpgi.00106.2023
miR-122-5p is involved in posttranscriptional regulation of the mitochondrial thiamin pyrophosphate transporter (SLC25A19) in pancreatic acinar cells
Abstract
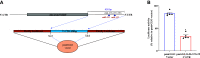
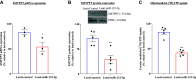
Thiamin (vitamin B1) plays a vital role in cellular energy metabolism/ATP production. Pancreatic acinar cells (PACs) obtain thiamin from circulation and convert it to thiamin pyrophosphate (TPP) in the cytoplasm. TPP is then taken up by the mitochondria via a carrier-mediated process that involves the mitochondrial TPP transporter (MTPPT; encoded by the gene SLC25A19). We have previously characterized different aspects of the mitochondrial carrier-mediated TPP uptake process, but nothing is known about its possible regulation at the posttranscriptional level. We address this issue in the current investigations focusing on the role of miRNAs in this regulation. First, we subjected the human (and rat) 3'-untranslated region (3'-UTR) of the SLC25A19 to three in-silico programs, and all have identified putative binding sites for miR-122-5p. Transfecting pmirGLO-hSLC25A19 3'-UTR into rat PAC AR42J resulted in a significant reduction in luciferase activity compared with cells transfected with pmirGLO-empty vector. Mutating as well as truncating the putative miR-122-5p binding sites in the hSLC25A19 3'-UTR led to abrogation of inhibition in luciferase activity in PAC AR42J. Furthermore, transfecting/transducing PAC AR42J and human primary PACs with mimic of miR-122-5p led to a significant inhibition in the level of expression of the MTPPT mRNA and protein as well as in mitochondrial carrier-mediated TPP uptake. Conversely, transfecting PAC AR42J with an inhibitor of miR-122-5p increased MTPPT expression and function. These findings show, for the first time, that expression and function of the MTPPT in PACs are subject to posttranscriptional regulation by miR-122-5p.NEW & NOTEWORTHY This study shows that the expression and function of mitochondrial TPP transporter (MTPPT) are subject to posttranscriptional regulation by miRNA-122-5p in pancreatic acinar cells.
Keywords: SLC25A19; miRNA; mitochondrial TPP transporter; posttranscriptional regulation.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures






References
-
- Berdanier CD. Advanced Nutrition-Micronutrients. New York: CRC Press, 1998.
-
- Gangolf M, Czerniecki J, Radermecker M, Detry O, Nisolle M, Jouan C, Martin D, Chantraine F, Lakaye B, Wins P, Grisar T, Bettendorff L. Thiamine status in humans and content of phosphorylated thiamine derivatives in biopsies and cultured cells. PLoS One 5: e13616, 2010. doi:10.1371/journal.pone.0013616. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

