Bridging semiempirical and ab initio QM/MM potentials by Gaussian process regression and its sparse variants for free energy simulation
- PMID: 37530109
- PMCID: PMC10400118
- DOI: 10.1063/5.0156327
Bridging semiempirical and ab initio QM/MM potentials by Gaussian process regression and its sparse variants for free energy simulation
Abstract
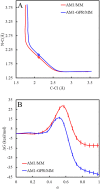
Free energy simulations that employ combined quantum mechanical and molecular mechanical (QM/MM) potentials at ab initio QM (AI) levels are computationally highly demanding. Here, we present a machine-learning-facilitated approach for obtaining AI/MM-quality free energy profiles at the cost of efficient semiempirical QM/MM (SE/MM) methods. Specifically, we use Gaussian process regression (GPR) to learn the potential energy corrections needed for an SE/MM level to match an AI/MM target along the minimum free energy path (MFEP). Force modification using gradients of the GPR potential allows us to improve configurational sampling and update the MFEP. To adaptively train our model, we further employ the sparse variational GP (SVGP) and streaming sparse GPR (SSGPR) methods, which efficiently incorporate previous sample information without significantly increasing the training data size. We applied the QM-(SS)GPR/MM method to the solution-phase SN2 Menshutkin reaction, NH3+CH3Cl→CH3NH3++Cl-, using AM1/MM and B3LYP/6-31+G(d,p)/MM as the base and target levels, respectively. For 4000 configurations sampled along the MFEP, the iteratively optimized AM1-SSGPR-4/MM model reduces the energy error in AM1/MM from 18.2 to 4.4 kcal/mol. Although not explicitly fitting forces, our method also reduces the key internal force errors from 25.5 to 11.1 kcal/mol/Å and from 30.2 to 10.3 kcal/mol/Å for the N-C and C-Cl bonds, respectively. Compared to the uncorrected simulations, the AM1-SSGPR-4/MM method lowers the predicted free energy barrier from 28.7 to 11.7 kcal/mol and decreases the reaction free energy from -12.4 to -41.9 kcal/mol, bringing these results into closer agreement with their AI/MM and experimental benchmarks.
© 2023 Author(s). Published under an exclusive license by AIP Publishing.
Conflict of interest statement
The authors have no conflicts to disclose.
Figures





References
-
- Field M. J., Bash P. A., and Karplus M., J. Comput. Chem. 11, 700–733 (1990). 10.1002/jcc.540110605 - DOI
-
- Singh U. C. and Kollman P. A., J. Comput. Chem. 7, 718–730 (1986). 10.1002/jcc.540070604 - DOI
-
- Gao J. and Thompson M. A., Combined Quantum Mechanical and Molecular Mechanical Methods (ACS Publications, 1998), Vol. 712.
Grants and funding
LinkOut - more resources
Full Text Sources

