Ticagrelor inverse agonist activity at the P2Y12 receptor is non-reversible versus its endogenous agonist adenosine 5´-diphosphate
- PMID: 37530222
- PMCID: PMC10953389
- DOI: 10.1111/bph.16204
Ticagrelor inverse agonist activity at the P2Y12 receptor is non-reversible versus its endogenous agonist adenosine 5´-diphosphate
Abstract
Background and purpose: Ticagrelor is labelled as a reversible, direct-acting platelet P2Y12 receptor (P2Y12 R) antagonist that is indicated clinically for the prevention of thrombotic events in patients with acute coronary syndrome (ACS). As with many antiplatelet drugs, ticagrelor therapy increases bleeding risk in patients, which may require platelet transfusion in emergency situations. The aim of this study was to further examine the reversibility of ticagrelor at the P2Y12 R.
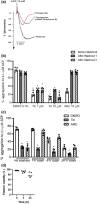
Experimental approach: Studies were performed in human platelets, with P2Y12 R-stimulated GTPase activity and platelet aggregation assessed. Cell-based bioluminescence resonance energy transfer (BRET) assays were undertaken to assess G protein-subunit activation downstream of P2Y12 R activation.
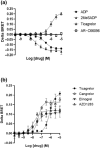
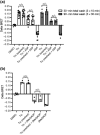
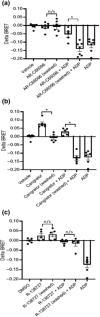
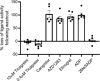
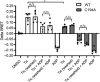
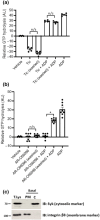
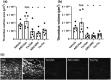
Key results: Initial studies revealed that a range of P2Y12 R ligands, including ticagrelor, displayed inverse agonist activity at P2Y12 R. Only ticagrelor was resistant to washout and, in human platelet and cell-based assays, washing failed to reverse ticagrelor-dependent inhibition of ADP-stimulated P2Y12 R function. The P2Y12 R agonist 2MeSADP, which was also resistant to washout, was able to effectively compete with ticagrelor. In silico docking revealed that ticagrelor and 2MeSADP penetrated more deeply into the orthosteric binding pocket of the P2Y12 R than other P2Y12 R ligands.
Conclusion and implications: Ticagrelor binding to P2Y12 R is prolonged and more akin to that of an irreversible antagonist, especially versus the endogenous P2Y12 R agonist ADP. This study highlights the potential clinical need for novel ticagrelor reversal strategies in patients with spontaneous major bleeding, and for bleeding associated with urgent invasive procedures.
Keywords: P2Y12 receptor; acute coronary syndrome; blood platelets; irreversibility; ticagrelor.
© 2023 The Authors. British Journal of Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
None.
Figures

References
-
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Southan, C. , Davies, J. A. , Abbracchio, M. P. , Alexander, W. , Al‐Hosaini, K. , Bäck, M. , Barnes, N. M. , … Bathgate, R. , Ye, R. D. (2021). The Concise Guide to PHARMACOLOGY 2021/22: G protein‐coupled receptors. British Journal of Pharmacology, 178, S27–S156. 10.1111/bph.15538 - DOI - PubMed
-
- Angiolillo, D. J. , Curzen, N. , Gurbel, P. , Vaitkus, P. , Lipkin, F. , Li, W. , Jakubowski, J. A. , Zettler, M. , Effron, M. B. , & Trenk, D. (2014). Pharmacodynamic evaluation of switching from ticagrelor to prasugrel in patients with stable coronary artery disease: Results of the SWAP‐2 study (Switching Anti Platelet‐2). Journal of the American College of Cardiology, 63(15), 1500–1509. 10.1016/j.jacc.2013.11.032 - DOI - PubMed
-
- Aungraheeta, R. , Conibear, A. , Butler, M. , Kelly, E. , Nylander, S. , Mumford, A. , & Mundell, S. J. (2016). Inverse agonism at the P2Y12 receptor and ENT1 transporter blockade contribute to platelet inhibition by ticagrelor. Blood, 128(23), 2717–2728. 10.1182/blood-2016-03-707844 - DOI - PMC - PubMed
-
- Bonaca, M. P. , Bhatt, D. L. , Cohen, M. , Steg, P. G. , Storey, R. F. , Jensen, E. C. , Magnani, G. , Bansilal, S. , Fish, M. P. , Im, K. , Bengtsson, O. , Ophuis, T. O. , Budaj, A. , Theroux, P. , Ruda, M. , Hamm, C. , Goto, S. , Spinar, J. , Nicolau, J. C. , … Sabatine, M. S. (2015). Long‐term use of ticagrelor in patients with prior myocardial infarction. The New England Journal of Medicine, 372(19), 1791–1800. 10.1056/NEJMoa1500857 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

