Using Data Science for Mechanistic Insights and Selectivity Predictions in a Non-Natural Biocatalytic Reaction
- PMID: 37530568
- PMCID: PMC10602048
- DOI: 10.1021/jacs.3c03639
Using Data Science for Mechanistic Insights and Selectivity Predictions in a Non-Natural Biocatalytic Reaction
Abstract
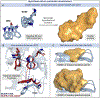
The study of non-natural biocatalytic transformations relies heavily on empirical methods, such as directed evolution, for identifying improved variants. Although exceptionally effective, this approach provides limited insight into the molecular mechanisms behind the transformations and necessitates multiple protein engineering campaigns for new reactants. To address this limitation, we disclose a strategy to explore the biocatalytic reaction space and garner insight into the molecular mechanisms driving enzymatic transformations. Specifically, we explored the selectivity of an "ene"-reductase, GluER-T36A, to create a data-driven toolset that explores reaction space and rationalizes the observed and predicted selectivities of substrate/mutant combinations. The resultant statistical models related structural features of the enzyme and substrate to selectivity and were used to effectively predict selectivity in reactions with out-of-sample substrates and mutants. Our approach provided a deeper understanding of enantioinduction by GluER-T36A and holds the potential to enhance the virtual screening of enzyme mutants.
Figures

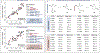


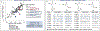

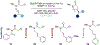
References
-
- Huffman MA; Fryszkowska A; Alvizo O; Borra-Garske M; Campos KR; Canada KA; Devine PN; Duan D; Forstater JH; Grosser ST; Halsey HM; Hughes GJ; Jo J; Joyce LA; Kolev JN; Liang J; Maloney KM; Mann BF; Marshall NM; McLaughlin M; Moore JC; Murphy GS; Nawrat CC; Nazor J; Novick S; Patel NR; Rodriguez-Granillo A; Robaire SA; Sherer EC; Truppo MD; Whittaker AM; Verma D; Xiao L; Xu Y; Yang H Design of an in Vitro Biocatalytic Cascade for the Manufacture of Islatravir. Science 2019, 366, 1255–1259. - PubMed
-
- Meghwanshi GK; Kaur N; Verma S; Dabi NK; Vashishtha A; Charan PD; Purohit P; Bhandari HS; Bhojak N; Kumar R Enzymes for Pharmaceutical and Therapeutic Applications. Biotechnol. Appl. Biochem 2020, 67, 586–601. - PubMed
-
- Duza MB; Mastan SA Microbial Enzymes and Their Applications—a Review. Indo Am. J. Pharm. Res 2013, 3, 651–657.
-
- Akyilmaz E; Yorganci E; Asav E Do Copper Ions Activate Tyrosinase Enzyme? A Biosensor Model for the Solution. Bioelectrochemistry 2010, 78, 155–160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

