A Conformationally Restricted Gold(III) Complex Elicits Antiproliferative Activity in Cancer Cells
- PMID: 37530672
- PMCID: PMC11268950
- DOI: 10.1021/acs.inorgchem.3c02066
A Conformationally Restricted Gold(III) Complex Elicits Antiproliferative Activity in Cancer Cells
Abstract
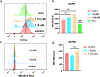
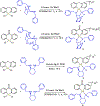
Diamine ligands are effective structural scaffolds for tuning the reactivity of transition-metal complexes for catalytic, materials, and phosphorescent applications and have been leveraged for biological use. In this work, we report the synthesis and characterization of a novel class of cyclometalated [C^N] Au(III) complexes bearing secondary diamines including a norbornane backbone, (2R,3S)-N2,N3-dibenzylbicyclo[2.2.1]heptane-2,3-diamine, or a cyclohexane backbone, (1R,2R)-N1,N2-dibenzylcyclohexane-1,2-diamine. X-ray crystallography confirms the square-planar geometry and chirality at nitrogen. The electronic character of the conformationally restricted norbornane backbone influences the electrochemical behavior with redox potentials of -0.8 to -1.1 V, atypical for Au(III) complexes. These compounds demonstrate promising anticancer activity, particularly, complex 1, which bears a benzylpyridine organogold framework, and supported by the bicyclic conformationally restricted diaminonorbornane, shows good potency in A2780 cells. We further show that a cellular response to 1 evokes reactive oxygen species (ROS) production and does not induce mitochondrial dysfunction. This class of complexes provides significant stability and reactivity for different applications in protein modification, catalysis, and therapeutics.
Conflict of interest statement
Conflicts of interest
The authors declare the following competing financial interest(s): Samuel G. Awuah (SGA) has patents pending to University of Kentucky Research Foundation. SGA serves on the advisory board and is Chief Science Officer for Phronesis AI.
Figures
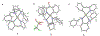
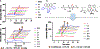

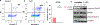

References
-
- Jürgens S; Scalcon V; Estrada-Ortiz N; Folda A; Tonolo F; Jandl C; Browne DL; Rigobello MP; Kühn FE; Casini A Exploring the ĈNĈ theme: Synthesis and biological properties of tridentate cyclometalated gold(III) complexes. Biorg. Med. Chem. 2017, 25 (20), 5452–5460. - PubMed
-
- Martín J; Gómez-Bengoa E; Genoux A; Nevado C Synthesis of Cyclometalated Gold(III) Complexes via Catalytic Rhodium to Gold(III) Transmetalation. Angew. Chem. Int. Ed. Engl. 2022, 61 (20), e202116755. - PubMed
-
- Gukathasan S; Awuah SG Synthetic Strategies for the Preparation of Gold-based Anticancer Agents. In Encyclopedia of Inorganic and Bioinorganic Chemistry, pp 1–32.
-
- Rocchigiani L; Bochmann M Recent Advances in Gold(III) Chemistry: Structure, Bonding, Reactivity, and Role in Homogeneous Catalysis. Chem. Rev. 2021, 121 (14), 8364–8451. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

