Beyond ingredients: Supramolecular structure of lipid droplets in infant formula affects metabolic and brain function in mouse models
- PMID: 37531323
- PMCID: PMC10395839
- DOI: 10.1371/journal.pone.0282816
Beyond ingredients: Supramolecular structure of lipid droplets in infant formula affects metabolic and brain function in mouse models
Erratum in
-
Correction: Beyond ingredients: Supramolecular structure of lipid droplets in infant formula affects metabolic and brain function in mouse models.PLoS One. 2025 Apr 1;20(4):e0321667. doi: 10.1371/journal.pone.0321667. eCollection 2025. PLoS One. 2025. PMID: 40168420 Free PMC article.
Abstract
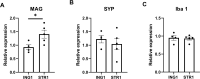
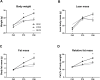
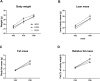
Human milk beneficially affects infant growth and brain development. The supramolecular structure of lipid globules in human milk i.e., large lipid globules covered by the milk fat globule membrane, is believed to contribute to this effect, in addition to the supply of functional ingredients. Three preclinical (mouse) experiments were performed to study the effects of infant formula mimicking the supramolecular structure of human milk lipid globules on brain and metabolic health outcomes. From postnatal day 16 to 42, mouse offspring were exposed to a diet containing infant formula with large, phospholipid-coated lipid droplets (structure, STR) or infant formula with the same ingredients but lacking the unique structural properties as observed in human milk (ingredient, ING). Subsequently, in Study 1, the fatty acid composition in liver and brain membranes was measured, and expression of hippocampal molecular markers were analyzed. In Study 2 and 3 adult (Western-style diet-induced) body fat accumulation and cognitive function were evaluated. Animals exposed to STR compared to ING showed improved omega-3 fatty acid accumulation in liver and brain, and higher expression of brain myelin-associated glycoprotein. Early exposure to STR reduced fat mass accumulation in adulthood; the effect was more pronounced in animals exposed to a Western-style diet. Additionally, mice exposed to STR demonstrated better memory performance later in life. In conclusion, early life exposure to infant formula containing large, phospholipid-coated lipid droplets, that are closer to the supramolecular structure of lipid globules in human milk, positively affects adult brain and metabolic health outcomes in pre-clinical animal models.
Copyright: © 2023 Oosting et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: This study was funded by Danone Nutricia Research. The funder provided support to the current study by covering research costs and in the form of salaries for authors AO, LH, SR and LS. The funder discloses a granted patent application using data that is also described in the submitted work (EP EP2753191A1 USE OF INFANT FORMULA WITH LARGE LIPID GLOBULES). GvD has collaborated with the funder on part of the submitted work for which his institution is compensated financially. The research is done in relation to development of a product by Danone, which is not yet released in (worldwide) markets. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures


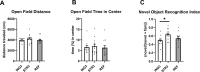
References
-
- Khan J, Vesel L, Bahl R, Martines JC. Timing of breastfeeding initiation and exclusivity of breastfeeding during the first month of life: effects on neonatal mortality and morbidity—a systematic review and meta-analysis. Matern Child Health J. 2015;19(3):468–79. doi: 10.1007/s10995-014-1526-8 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

