Outcome prediction in pediatric fever in neutropenia: Development of clinical decision rules and external validation of published rules based on data from the prospective multicenter SPOG 2015 FN definition study
- PMID: 37531403
- PMCID: PMC10395874
- DOI: 10.1371/journal.pone.0287233
Outcome prediction in pediatric fever in neutropenia: Development of clinical decision rules and external validation of published rules based on data from the prospective multicenter SPOG 2015 FN definition study
Abstract
Background: Fever in neutropenia (FN) remains a serious complication of childhood cancer therapy. Clinical decision rules (CDRs) are recommended to help distinguish between children at high and low risk of severe infection. The aim of this analysis was to develop new CDRs for three different outcomes and to externally validate published CDRs.
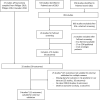
Procedure: Children undergoing chemotherapy for cancer were observed in a prospective multicenter study. CDRs predicting low from high risk infection regarding three outcomes (bacteremia, serious medical complications (SMC), safety relevant events (SRE)) were developed from multivariable regression models. Their predictive performance was assessed by internal cross-validation. Published CDRs suitable for validation were identified by literature search. Parameters of predictive performance were compared to assess reproducibility.
Results: In 158 patients recruited between April 2016 and August 2018, 360 FN episodes were recorded, including 56 (16%) with bacteremia, 30 (8%) with SMC and 72 (20%) with SRE. The CDRs for bacteremia and SRE used four characteristics (type of malignancy, severely reduced general condition, leucocyte count <0.3 G/L, bone marrow involvement), the CDR for SMC two characteristics (severely reduced general condition and platelet count <50 G/L). Eleven published CDRs were analyzed. Six CDRs showed reproducibility, but only one in both sensitivity and specificity.
Conclusions: This analysis developed CDRs predicting bacteremia, SMC or SRE at presentation with FN. In addition, it identified six published CDRs that show some reproducibility. Validation of CDRs is fundamental to find the best balance between sensitivity and specificity, and will help to further improve management of FN.
Copyright: © 2023 Santschi et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Predicting fever in neutropenia with safety-relevant events in children undergoing chemotherapy for cancer: The prospective multicenter SPOG 2015 FN Definition Study.Pediatr Blood Cancer. 2021 Dec;68(12):e29253. doi: 10.1002/pbc.29253. Epub 2021 Jul 26. Pediatr Blood Cancer. 2021. PMID: 34310027
-
External Validation of Six Pediatric Fever and Neutropenia Clinical Decision Rules.Pediatr Infect Dis J. 2018 Apr;37(4):329-335. doi: 10.1097/INF.0000000000001777. Pediatr Infect Dis J. 2018. PMID: 28877157
-
Predicting bacteremia in children with cancer and fever in chemotherapy-induced neutropenia: results of the prospective multicenter SPOG 2003 FN study.Pediatr Infect Dis J. 2011 Jul;30(7):e114-9. doi: 10.1097/INF.0b013e318215a290. Pediatr Infect Dis J. 2011. PMID: 21394050
-
Predicting the risk of severe infection in children with chemotherapy-induced febrile neutropenia.Curr Opin Hematol. 2012 Jan;19(1):39-43. doi: 10.1097/MOH.0b013e32834da951. Curr Opin Hematol. 2012. PMID: 22123661 Review.
-
Emergency management of fever and neutropenia in children with cancer: A review.Am J Emerg Med. 2021 Dec;50:693-698. doi: 10.1016/j.ajem.2021.09.055. Epub 2021 Sep 28. Am J Emerg Med. 2021. PMID: 34879488 Review.
References
-
- Brack E, Bodmer N, Simon A, Leibundgut K, Kuhne T, Niggli FK, et al.. First-day step-down to oral outpatient treatment versus continued standard treatment in children with cancer and low-risk fever in neutropenia. A randomized controlled trial within the multicenter SPOG 2003 FN study. Pediatr Blood Cancer 2012, 59(3):423–430. - PubMed
-
- Lehrnbecher T, Robinson P, Ammann RA, Fisher B, Patel P, Phillips R, et al.. Guideline for the Management of Fever and Neutropenia in Pediatric Patients With Cancer and Hematopoietic Cell Transplantation Recipients: 2023 Update. J Clin Oncol 2023, 20;41(9):1774–1785. doi: 10.1200/JCO.22.02224 - DOI - PMC - PubMed
-
- Lavieri L, Koenig C, Bodmer N, Agyeman PKA, Scheinemann K, Ansari M, et al.. Predicting fever in neutropenia with safety-relevant events in children undergoing chemotherapy for cancer: The prospective multicenter SPOG 2015 FN Definition Study. Pediatr Blood Cancer 2021, 68(12):e29253. doi: 10.1002/pbc.29253 - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

