Exportin 1 inhibition prevents neuroendocrine transformation through SOX2 down-regulation in lung and prostate cancers
- PMID: 37531417
- PMCID: PMC10777207
- DOI: 10.1126/scitranslmed.adf7006
Exportin 1 inhibition prevents neuroendocrine transformation through SOX2 down-regulation in lung and prostate cancers
Abstract
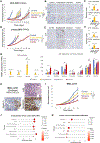
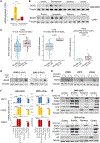
In lung and prostate adenocarcinomas, neuroendocrine (NE) transformation to an aggressive derivative resembling small cell lung cancer (SCLC) is associated with poor prognosis. We previously described dependency of SCLC on the nuclear transporter exportin 1. Here, we explored the role of exportin 1 in NE transformation. We observed up-regulated exportin 1 in lung and prostate pretransformation adenocarcinomas. Exportin 1 was up-regulated after genetic inactivation of TP53 and RB1 in lung and prostate adenocarcinoma cell lines, accompanied by increased sensitivity to the exportin 1 inhibitor selinexor in vitro. Exportin 1 inhibition prevented NE transformation in different TP53/RB1-inactivated prostate adenocarcinoma xenograft models that acquire NE features upon treatment with the aromatase inhibitor enzalutamide and extended response to the EGFR inhibitor osimertinib in a lung cancer transformation patient-derived xenograft (PDX) model exhibiting combined adenocarcinoma/SCLC histology. Ectopic SOX2 expression restored the enzalutamide-promoted NE phenotype on adenocarcinoma-to-NE transformation xenograft models despite selinexor treatment. Selinexor sensitized NE-transformed lung and prostate small cell carcinoma PDXs to standard cytotoxics. Together, these data nominate exportin 1 inhibition as a potential therapeutic target to constrain lineage plasticity and prevent or treat NE transformation in lung and prostate adenocarcinoma.
Figures



References
-
- Marcoux N, Gettinger SN, O’Kane G, Arbour KC, Neal JW, Husain H, Evans TL, Brahmer JR, Muzikansky A, Bonomi PD, del Prete S, Wurtz A, Farago AF, DiasSantagata D, Mino-Kenudson M, Reckamp KL, Yu HA, Wakelee HA, Shepherd FA, Piotrowska Z, Sequist LV, EGFR-mutant adenocarcinomas that transform to small-cell lung cancer and other neuroendocrine carcinomas: Clinical outcomes. J. Clin. Oncol. 37, 278–285 (2019). - PMC - PubMed
-
- Aggarwal R, Huang J, Alumkal JJ, Zhang L, Feng FY, Thomas GV, Weinstein AS, Friedl V, Zhang C, Witte ON, Lloyd P, Gleave M, Evans CP, Youngren J, Beer TM, Rettig M, Wong CK, True L, Foye A, Playdle D, Ryan CJ, Lara P, Chi KN, Uzunangelov V, Sokolov A, Newton Y, Beltran H, Demichelis F, Rubin MA, Stuart JM, Small EJ, Clinical and genomic characterization of treatment-emergent small-cell neuroendocrine prostate cancer: A multi-institutional prospective study. J. Clin. Oncol. 36, 2492–2503 (2018). - PMC - PubMed
-
- Quintanal-Villalonga A, Taniguchi H, Zhan YA, Hasan MM, Chavan SS, Meng F, Uddin F, Manoj P, Donoghue MTA, Won HH, Chan JM, Ciampricotti M, Chow A, Offin M, Chang JC, Ray-Kirton J, Tischfield SE, Egger J, Bhanot UK, Linkov I, Asher M, Sinha S, Silber J, Iacobuzio-Donahue CA, Roehrl MH, Hollmann TJ, Yu HA, Qiu J, de Stanchina E, Baine MK, Rekhtman N, Poirier JT, Loomis B, Koche RP, Rudin CM, Sen T, Multi-omic analysis of lung tumors defines pathways activated in neuroendocrine transformation. Cancer Discov. 11, 3028–3047 (2021). - PMC - PubMed
-
- Rudin CM, Durinck S, Stawiski EW, Poirier JT, Modrusan Z, Shames DS, Bergbower EA, Guan Y, Shin J, Guillory J, Rivers CS, Foo CK, Bhatt D, Stinson J, Gnad F, Haverty PM, Gentleman R, Chaudhuri S, Janakiraman V, Jaiswal BS, Parikh C, Yuan W, Zhang Z, Koeppen H, Wu TD, Stern HM, Yauch RL, Huffman KE, Paskulin DD, Illei PB, Varella-Garcia M, Gazdar AF, De Sauvage FJ, Bourgon R, Minna JD, Brock MV, Seshagiri S, Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat. Genet. 44, 1111–1116 (2012). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

