Inducible nonhuman primate models of retinal degeneration for testing end-stage therapies
- PMID: 37531424
- PMCID: PMC10396314
- DOI: 10.1126/sciadv.adg8163
Inducible nonhuman primate models of retinal degeneration for testing end-stage therapies
Abstract
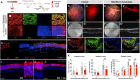
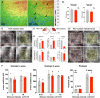
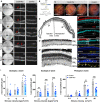
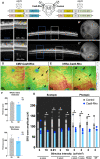
The anatomical differences between the retinas of humans and most animal models pose a challenge for testing novel therapies. Nonhuman primate (NHP) retina is anatomically closest to the human retina. However, there is a lack of relevant NHP models of retinal degeneration (RD) suitable for preclinical studies. To address this unmet need, we generated three distinct inducible cynomolgus macaque models of RD. We developed two genetically targeted strategies using optogenetics and CRISPR-Cas9 to ablate rods and mimic rod-cone dystrophy. In addition, we created an acute model by physical separation of the photoreceptors and retinal pigment epithelium using a polymer patch. Among the three models, the CRISPR-Cas9-based approach was the most advantageous model in view of recapitulating disease-specific features and its ease of implementation. The acute model, however, resulted in the fastest degeneration, making it the most relevant model for testing end-stage vision restoration therapies such as stem cell transplantation.
Figures



References
-
- A. P. Marques, J. Ramke, J. Cairns, T. Butt, J. H. Zhang, I. Jones, M. Jovic, A. Nandakumar, H. Faal, H. Taylor, A. Bastawrous, T. Braithwaite, S. Resnikoff, P. T. Khaw, R. Bourne, I. Gordon, K. Frick, M. J. Burton, The economics of vision impairment and its leading causes: A systematic review. eClinicalMedicine 46, 101354 (2022). - PMC - PubMed
-
- B. Roska, J. A. Sahel, Restoring vision. Nature 557, 359–367 (2018). - PubMed
-
- A. Hendrickson, A morphological comparison of foveal development in man and monkey. Eye 6, 136–144 (1992). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

