A Systematic Review and Meta-Analysis of Interventions to Promote Adjuvant Endocrine Therapy Adherence Among Breast Cancer Survivors
- PMID: 37531593
- PMCID: PMC10553065
- DOI: 10.1200/JCO.23.00697
A Systematic Review and Meta-Analysis of Interventions to Promote Adjuvant Endocrine Therapy Adherence Among Breast Cancer Survivors
Abstract
Purpose: Adjuvant endocrine therapy (AET) adherence among breast cancer survivors is often suboptimal, leading to higher cancer recurrence and mortality. Intervention studies to promote AET adherence have burgeoned, more than doubling in number since this literature was last reviewed. The current aim is to provide an up-to-date systematic review and meta-analysis of interventions to enhance AET adherence and to identify strengths and limitations of existing interventions to inform future research and clinical care.
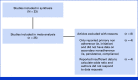
Methods: Systematic searches were conducted in three electronic databases. Studies were included in the systematic review if they examined an intervention for promoting AET adherence among breast cancer survivors. Studies were further included in the meta-analyses if they examined a measure of AET adherence (defined as compliance or persistence beyond initiation) and reported (or provided upon request) sufficient information to calculate an effect size.
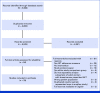
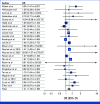
Results: Of 5,045 unique records, 33 unique studies representing 375,951 women met inclusion criteria for the systematic review. Interventions that educated patients about how to manage side effects generally failed to improve AET adherence, whereas policy changes that lowered AET costs consistently improved adherence. Medication reminders, communication, and psychological/coping strategies showed varied efficacy. Of the 33 studies that met the inclusion criteria for the systematic review, 25 studies representing 367,873 women met inclusion criteria for the meta-analysis. The meta-analysis showed statistically significant effects of the adherence interventions overall relative to study-specified control conditions (number of studies [k] = 25; odds ratio, 1.412; 95% CI, 1.183 to 1.682; P = .0001). Subgroup analyses showed that there were no statistically significant differences in effect sizes by study design (randomized controlled trial v other), publication year, directionality of the intervention (unidirectional v bidirectional contact), or intervention type.
Conclusion: To our knowledge, this is the first known meta-analysis to demonstrate a significant effect for interventions to promote AET adherence. The systematic review revealed that lowering medication costs and a subgroup of psychosocial and reminder interventions showed the most promise, informing future research, policy, and clinical directions.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures
References
-
- Burstein HJ, Lacchetti C, Anderson H, et al. : Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: ASCO clinical practice guideline focused update. J Clin Oncol 37:423-438, 2019 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

