A Phase 2 Extension Study Evaluating the Immunogenicity, Safety, and Tolerability of 3 or 4 Doses of a Clostridioides difficile Vaccine in Healthy US Adults Aged 65 to 85 Years
- PMID: 37531657
- PMCID: PMC10873164
- DOI: 10.1093/infdis/jiad307
A Phase 2 Extension Study Evaluating the Immunogenicity, Safety, and Tolerability of 3 or 4 Doses of a Clostridioides difficile Vaccine in Healthy US Adults Aged 65 to 85 Years
Abstract
Background: This phase 2 extension explored the long-term antibody persistence of an investigational Clostridioides difficile vaccine and the safety, tolerability, and immunogenicity of dose 4 approximately 12 months post-dose 3.
Methods: One year post-dose 3, healthy US 65- to 85-year-olds (N = 300) were randomized to dose 4 of vaccine at previously received antigen levels (100 or 200 μg) or placebo. Assessments included safety and percentages of participants achieving neutralizing antibody titers above prespecified thresholds (≥219 and ≥2586 neutralization units/mL for toxins A and B, respectively).
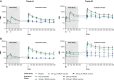
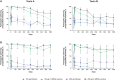
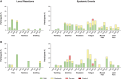
Results: In participants previously given three 200-µg doses and placebo in the extension, toxin A and B neutralizing antibodies were above prevaccination levels 48 months post-dose 3 (36 months after placebo); 24.0% and 26.0% had toxin A and B antibodies at or above prespecified thresholds, respectively. Neutralizing antibodies increased post-dose 4 (12 months post-dose 3) and persisted to 36 months post-dose 4. Thirty days post-dose 4, all participants had toxin A and 86.5% to 100% had toxin B titers at or above prespecified thresholds. Local reactions were more frequent in vaccine recipients. Systemic and adverse event frequencies were similar across groups.
Conclusions: C difficile vaccine immune responses persisted 48 months post-dose 3. Dose 4 was immunogenic and well tolerated, supporting continued development. Clinical Trials Registration. ClinicalTrials.gov NCT02561195.
Keywords: Clostridioides difficile infection; United States; adults; nosocomial diarrhea; toxoid vaccine.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest . S. R., N. K., P. L., M. W. P., L. B., A. S. A., W. C. G., K. U. J., S. P. L., and C. W. are current or former employees of Pfizer Inc and may hold stock and/or stock options. J. P. received support from Pfizer as a study investigator. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
-
- Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med 2015; 372:1539–48. - PubMed
-
- Kelly CP, Pothoulakis C, LaMont JT. Clostridium difficile colitis. N Engl J Med 1994; 330:257–62. - PubMed
-
- Bauer MP, Notermans DW, van Benthem BH, et al. . Clostridium difficile infection in Europe: a hospital-based survey. Lancet 2011; 377:63–73. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

