Risk of Subsequent Respiratory Virus Detection After Primary Virus Detection in a Community Household Study-King County, Washington, 2019-2021
- PMID: 37531658
- PMCID: PMC10873185
- DOI: 10.1093/infdis/jiad305
Risk of Subsequent Respiratory Virus Detection After Primary Virus Detection in a Community Household Study-King County, Washington, 2019-2021
Abstract
Background: The epidemiology of respiratory viral infections is complex. How infection with one respiratory virus affects risk of subsequent infection with the same or another respiratory virus is not well described.
Methods: From October 2019 to June 2021, enrolled households completed active surveillance for acute respiratory illness (ARI), and participants with ARI self-collected nasal swab specimens; after April 2020, participants with ARI or laboratory-confirmed severe acute respiratory syndrome coronavirus 2 and their household members self-collected nasal swab specimens. Specimens were tested using multiplex reverse-transcription polymerase chain reaction for respiratory viruses. A Cox regression model with a time-dependent covariate examined risk of subsequent detections following a specific primary viral detection.
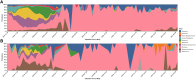
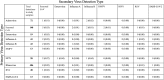
Results: Rhinovirus was the most frequently detected pathogen in study specimens (406 [9.5%]). Among 51 participants with multiple viral detections, rhinovirus to seasonal coronavirus (8 [14.8%]) was the most common viral detection pairing. Relative to no primary detection, there was a 1.03-2.06-fold increase in risk of subsequent virus detection in the 90 days after primary detection; risk varied by primary virus: human parainfluenza virus, rhinovirus, and respiratory syncytial virus were statistically significant.
Conclusions: Primary virus detection was associated with higher risk of subsequent virus detection within the first 90 days after primary detection.
Keywords: coinfection; household transmission; respiratory viral infection; seasonal epidemic; viral interference.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest . E. J. C. received honoraria from Providence Regional Medical Center in Everett, Washington, for presentations on coronavirus disease 2019 and received a travel award from the Infectious Diseases Society of America to attend IDWeek 2022. J. A. E. reports consulting with AstraZeneca, Meissa Vaccines, and Sanofi Pasteur, as well as research support from AstraZeneca, GlaxoSmithKline, Merck, and Pfizer. H. Y. C. reports consulting with Ellume, Pfizer, the Bill and Melinda Gates Foundation, Glaxo Smith Kline, and Merck. She has received research funding from Gates Ventures and Sanofi Pasteur and support and reagents from Ellume and Cepheid, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

References
-
- Rha B, Curns AT, Lively JY, et al. . Respiratory syncytial virus-associated hospitalizations among young children: 2015–2016. Pediatrics 2020; 146:e20193611. - PubMed
-
- Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 2005; 352:1749–59. - PubMed
-
- Fendrick AM, Monto AS, Nightengale B, Sarnes M. The economic burden of non-influenza-related viral respiratory tract infection in the United States. Arch Intern Med 2003; 163:487–94. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

