EP1 activation inhibits doxorubicin-cardiomyocyte ferroptosis via Nrf2
- PMID: 37531930
- PMCID: PMC10400469
- DOI: 10.1016/j.redox.2023.102825
EP1 activation inhibits doxorubicin-cardiomyocyte ferroptosis via Nrf2
Abstract
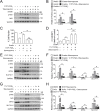
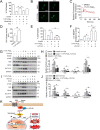
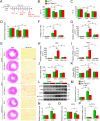
Chemotherapeutic agents, such as doxorubicin (DOX), may cause cardiomyopathy, even life-threatening arrhythmias in cancer patients. Ferroptosis-an iron-dependent oxidative form of programmed necrosis, plays a pivotal role in DOX-induced cardiomyopathy (DIC). Prostaglandins (PGs) are bioactive signaling molecules that profoundly modulate cardiac performance in both physiologic and pathologic conditions. Here, we found that PGE2 production and its E-prostanoid 1 receptor (EP1) expression were upregulated in erastin (a ferroptosis inducer) or DOX-treated cardiomyocytes. EP1 inhibition markedly aggravated erastin or DOX-induced cardiomyocyte ferroptosis, whereas EP1 activation exerted opposite effect. Genetic depletion of EP1 in cardiomyocytes worsens DOX-induced cardiac injury in mice, which was efficiently rescued by the ferroptosis inhibitor Ferrostatin-1 (Fer-1). Mechanistically, EP1 activation protected cardiomyocytes from DOX-induced ferroptosis by promoting nuclear factor erythroid 2-related factor 2 (Nrf2)-driven anti-oxidative gene expression, such as glutathione peroxidase 4 (GPX4) and solute carrier family 7 member 11 (SLC7A11). EP1 was coupled with Gαq to elicit intracellular Ca2+ flux and activate the PKC/Nrf2 cascade in ferroptotic cardiomyocytes. EP1 activation also prevents DOX-induced ferroptosis in human cardiomyocytes. Thus, PGE2/EP1 axis protects cardiomyocytes from DOX-induced ferroptosis by activating PKC/Nrf2 signaling and activation of EP1 may represent an attractive strategy for DIC prevention and treatment.
Keywords: Cardiomyocyte ferroptosis; DOX-induced cardiomyopathy; EP1; Nrf2.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that there is no conflict of interest
Figures




References
-
- Ewer M.S., Ewer S.M. Cardiotoxicity of anticancer treatments. Nat. Rev. Cardiol. 2015;12(11):620. - PubMed
-
- Raisi-Estabragh Z., Kobo O., Freeman P., Petersen S.E., Kolman L., Miller R.J.H., Roguin A., Van Spall H.G.C., Vuong J., Yang E.H., Mamas M.A. Temporal trends in disease-specific causes of cardiovascular mortality amongst patients with cancer in the USA between 1999 and 2019. Eur Heart J Qual Care Clin Outcomes. 2022;9(1):54–63. - PMC - PubMed
-
- Singal P.K., Iliskovic N. Doxorubicin-induced cardiomyopathy. N. Engl. J. Med. 1998;339(13):900–905. - PubMed
-
- Zhang S., Liu X.B., Bawa-Khalfe T., Lu L.S., Lyu Y.L., Liu L.F., Yeh E.T.H. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat. Med. 2012;18(11):1639–1642. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

