The seizure severity score: a quantitative tool for comparing seizures and their response to therapy
- PMID: 37531949
- PMCID: PMC11250994
- DOI: 10.1088/1741-2552/aceca1
The seizure severity score: a quantitative tool for comparing seizures and their response to therapy
Abstract
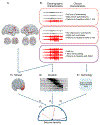
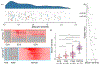
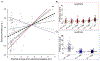
Objective.Epilepsy is a neurological disorder characterized by recurrent seizures which vary widely in severity, from clinically silent to prolonged convulsions. Measuring severity is crucial for guiding therapy, particularly when complete control is not possible. Seizure diaries, the current standard for guiding therapy, are insensitive to the duration of events or the propagation of seizure activity across the brain. We present a quantitative seizure severity score that incorporates electroencephalography (EEG) and clinical data and demonstrate how it can guide epilepsy therapies.Approach.We collected intracranial EEG and clinical semiology data from 54 epilepsy patients who had 256 seizures during invasive, in-hospital presurgical evaluation. We applied an absolute slope algorithm to EEG recordings to identify seizing channels. From this data, we developed a seizure severity score that combines seizure duration, spread, and semiology using non-negative matrix factorization. For validation, we assessed its correlation with independent measures of epilepsy burden: seizure types, epilepsy duration, a pharmacokinetic model of medication load, and response to epilepsy surgery. We investigated the association between the seizure severity score and preictal network features.Main results.The seizure severity score augmented clinical classification by objectively delineating seizure duration and spread from recordings in available electrodes. Lower preictal medication loads were associated with higher seizure severity scores (p= 0.018, 97.5% confidence interval = [-1.242, -0.116]) and lower pre-surgical severity was associated with better surgical outcome (p= 0.042). In 85% of patients with multiple seizure types, greater preictal change from baseline was associated with higher severity.Significance.We present a quantitative measure of seizure severity that includes EEG and clinical features, validated on gold standard in-patient recordings. We provide a framework for extending our tool's utility to ambulatory EEG devices, for linking it to seizure semiology measured by wearable sensors, and as a tool to advance data-driven epilepsy care.
Keywords: epilepsy; intracranial EEG; seizure spread.
© 2023 IOP Publishing Ltd.
Conflict of interest statement
Conflict of interest
Erin Conrad performs consulting work for Epiminder, an EEG device company. The remaining authors have no conflicts of interest.
Figures
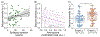
References
-
- Kwan P, Schachter SC and Brodie MJ 2011. Drug-resistant epilepsy New Engl. J. Med 365 919–26 - PubMed
-
- Cook MJ et al. 2013. Prediction of seizure likelihood with a long-term, implanted seizure advisory system in patients with drug-resistant epilepsy: a first-in-man study Lancet Neurol. 12 563–71 - PubMed
-
- Fisher RS, Blum DE, DiVentura B, Vannest J, Hixson JD, Moss R, Herman ST, Fureman BE and French JA 2012. Seizure diaries for clinical research and practice: limitations and future prospects Epilepsy Behav. 24 304–10 - PubMed
-
- Haut SR, Hall CB, LeValley AJ and Lipton RB 2007. Can patients with epilepsy predict their seizures? Neurology 68 262–6 - PubMed
-
- Szaflarski M, Meckler JM, Privitera MD and Szaflarski JP 2006. Quality of life in medication-resistant epilepsy: the effects of patient’s age, age at seizure onset, and disease duration Epilepsy Behav. 8 547–51 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
