Aberrant accumulation of age- and disease-associated factors following neural probe implantation in a mouse model of Alzheimer's disease
- PMID: 37531953
- PMCID: PMC10594264
- DOI: 10.1088/1741-2552/aceca5
Aberrant accumulation of age- and disease-associated factors following neural probe implantation in a mouse model of Alzheimer's disease
Abstract
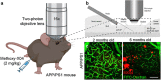
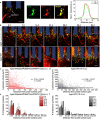
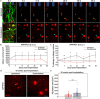
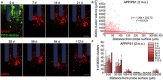
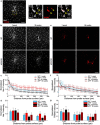
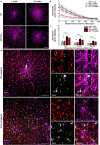
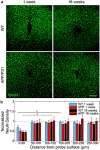
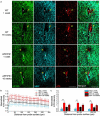
Objective. Electrical stimulation has had a profound impact on our current understanding of nervous system physiology and provided viable clinical options for addressing neurological dysfunction within the brain. Unfortunately, the brain's immune suppression of indwelling microelectrodes currently presents a major roadblock in the long-term application of neural recording and stimulating devices. In some ways, brain trauma induced by penetrating microelectrodes produces similar neuropathology as debilitating brain diseases, such as Alzheimer's disease (AD), while also suffering from end-stage neuron loss and tissue degeneration. The goal of the present study was to understand whether there may be any parallel mechanisms at play between brain injury from chronic microelectrode implantation and those of neurodegenerative disorder.Approach. We used two-photon microscopy to visualize the accumulation, if any, of age- and disease-associated factors around chronically implanted electrodes in both young and aged mouse models of AD.Main results. We determined that electrode injury leads to aberrant accumulation of lipofuscin, an age-related pigment, in wild-type and AD mice alike. Furthermore, we reveal that chronic microelectrode implantation reduces the growth of pre-existing Alzheimer's plaques while simultaneously elevating amyloid burden at the electrode-tissue interface. Lastly, we uncover novel spatial and temporal patterns of glial reactivity, axonal and myelin pathology, and neurodegeneration related to neurodegenerative disease around chronically implanted microelectrodes.Significance. This study offers multiple novel perspectives on the possible neurodegenerative mechanisms afflicting chronic brain implants, spurring new potential avenues of neuroscience investigation and design of more targeted therapies for improving neural device biocompatibility and treatment of degenerative brain disease.
Keywords: amyloid beta; foreign body response; lysosome; metabolism; neural interfaces; tauopathy.
Creative Commons Attribution license.
Figures
Update of
-
Aberrant accumulation of age- and disease-associated factors following neural probe implantation in a mouse model of Alzheimer's disease.bioRxiv [Preprint]. 2023 Feb 12:2023.02.11.528131. doi: 10.1101/2023.02.11.528131. bioRxiv. 2023. Update in: J Neural Eng. 2023 Sep 01;20(4). doi: 10.1088/1741-2552/aceca5. PMID: 36891286 Free PMC article. Updated. Preprint.
References
-
- Michelson N J, Vazquez A L, Eles J R, Salatino J W, Purcell E K, Williams J J, Cui X T, Kozai T D Y. Multi-scale, multi-modal analysis uncovers complex relationship at the brain tissue-implant neural interface: new emphasis on the biological interface. J. Neural Eng. 2018;15:033001. doi: 10.1088/1741-2552/aa9dae. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
