The proportions of term or late preterm births after exposure to early antenatal corticosteroids, and outcomes: systematic review and meta-analysis of 1.6 million infants
- PMID: 37532269
- PMCID: PMC10394681
- DOI: 10.1136/bmj-2023-076035
The proportions of term or late preterm births after exposure to early antenatal corticosteroids, and outcomes: systematic review and meta-analysis of 1.6 million infants
Abstract
Objective: To systematically review the proportions of infants with early exposure to antenatal corticosteroids but born at term or late preterm, and short term and long term outcomes.
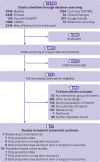
Design: Systematic review and meta-analyses.
Data sources: Eight databases searched from 1 January 2000 to 1 February 2023, reflecting recent perinatal care, and references of screened articles.
Eligibility criteria for selecting studies: Randomised controlled trials and population based cohort studies with data on infants with early exposure to antenatal corticosteroids (<34 weeks) but born at term (≥37 weeks), late preterm (34-36 weeks), or term/late preterm combined.
Data extraction and synthesis: Two reviewers independently screened titles, abstracts, and full text articles and assessed risk of bias (Cochrane risk of bias tool for randomised controlled trials and Newcastle-Ottawa scale for population based studies). Reviewers extracted data on populations, exposure to antenatal corticosteroids, and outcomes. The authors analysed randomised and cohort data separately, using random effects meta-analyses.
Main outcome measures: The primary outcome was the proportion of infants with early exposure to antenatal corticosteroids but born at term. Secondary outcomes included the proportions of infants born late preterm or term/late preterm combined after early exposure to antenatal corticosteroids and short term and long term outcomes versus non-exposure for the three gestational time points (term, late preterm, term/late preterm combined).
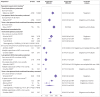
Results: Of 14 799 records, the reviewers screened 8815 non-duplicate titles and abstracts and assessed 713 full text articles. Seven randomised controlled trials and 10 population based cohort studies (1.6 million infants total) were included. In randomised controlled trials and population based data, ∼40% of infants with early exposure to antenatal corticosteroids were born at term (low or very low certainty). Among children born at term, early exposure to antenatal corticosteroids versus no exposure was associated with increased risks of admission to neonatal intensive care (adjusted odds ratio 1.49, 95% confidence interval 1.19 to 1.86, one study, 5330 infants, very low certainty; unadjusted relative risk 1.69, 95% confidence interval 1.51 to 1.89, three studies, 1 176 022 infants, I2=58%, τ2=0.01, low certainty), intubation (unadjusted relative risk 2.59, 1.39 to 4.81, absolute effect 7 more per 1000, 95% confidence interval from 2 more to 16 more, one study, 8076 infants, very low certainty, one study, 8076 infants, very low certainty), reduced head circumference (adjusted mean difference -0.21, 95% confidence interval -0.29 to -0.13, one study, 183 325 infants, low certainty), and any long term neurodevelopmental or behavioural disorder in population based studies (eg, any neurodevelopmental or behavioural disorder in children born at term, adjusted hazard ratio 1.47, 95% confidence interval 1.36 to 1.60, one study, 641 487 children, low certainty).
Conclusions: About 40% of infants exposed to early antenatal corticosteroids were born at term, with associated adverse short term and long term outcomes (low or very low certainty), highlighting the need for caution when considering antenatal corticosteroids.
Systematic review registration: PROSPERO CRD42022360079.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- National Institute for Health and Care Excellence. Preterm labour and birth. 2022. NICE guideline, No 25 . https://www.nice.org.uk/guidance/ng25 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
