Brentuximab vedotin with AVD for stage II-IV HIV-related Hodgkin lymphoma (AMC 085): phase 2 results from an open-label, single arm, multicentre phase 1/2 trial
- PMID: 37532416
- PMCID: PMC10859222
- DOI: 10.1016/S2352-3026(23)00157-6
Brentuximab vedotin with AVD for stage II-IV HIV-related Hodgkin lymphoma (AMC 085): phase 2 results from an open-label, single arm, multicentre phase 1/2 trial
Abstract
Background: Brentuximab vedotin in combination with doxorubicin, vinblastine, and dacarbazine (AVD) is approved in the upfront setting for advanced stage classical Hodgkin lymphoma (cHL). People living with HIV have been excluded from these studies. We aimed to understand the activity and safety of brentuximab vedotin-AVD in people living with HIV diagnosed with Hodgkin lymphoma, while focusing on HIV disease parameters and antiretroviral therapy (ART) interactions.
Methods: We present the phase 2 portion of a multicentre phase 1/2 study. Eligible patients were 18 years or older, had untreated stage II-IV HIV-associated cHL (HIV-cHL), a Karnofsky performance status of more than 30%, a CD4+ T-cell count of 50 cells per μL or more, were required to take ART, and were not on strong CYP3A4 or P-glycoprotein inhibitors. Patients were treated intravenously with 1·2 mg/kg of brentuximab vedotin (recommended phase 2 dose) with standard doses of AVD for six cycles on days 1 and 15 of a 28-day cycle. The primary endpoint of the phase 2 portion was 2-year progression-free survival (PFS), assessed in all eligible participants who began treatment. Accrual has been completed. This trial is registered at ClinicalTrials.gov, NCT01771107.
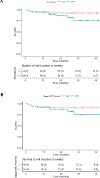
Findings: Between March 8, 2013, and March 7, 2019, 41 patients received study therapy with a median follow up of 29 months (IQR 16-38). 34 (83%) of 41 patients presented with stage III-IV and seven (17%) with stage II unfavourable HIV-cHL. 37 (90%) of 41 patients completed therapy, all 37 of whom achieved complete response. The 2-year PFS was 87% (95% CI 71-94) and the overall survival was 92% (78-97). The most common grade 3 or worse adverse events were peripheral sensory neuropathy (four [10%] of 41 patients), neutropenia (18 [44%]), and febrile neutropenia (five [12%]). One treatment-related death was reported, due to infection.
Interpretation: Brentuximab vedotin-AVD was highly active and had a tolerable adverse event rate in HIV-cHL and is an important therapeutic option for people with HIV-cHL. The complete reponse rate is encouraging and is possibly related to a unique aspect of HIV-cHL biology. Upcoming 5-year data will evaluate the sustainability of the outcomes obtained.
Funding: National Institutes of Health and National Cancer Institute.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests AN has an MSK core grant National Institutes of Health (NIH) P30 CA008748 and NIH grant PO1 1568 G TA390. MAR is supported by the Johns Hopkins University Core Grant P30CA006973, contracts with Cullinan Apollo and RenovoRx, and is a founder and serves on the Board of Directors of Geminus Therapeutics. EGR is the co-chair of the National Comprehensive Cancer Network panel of Cancer in Persons With HIV and Kaposi Sarcoma, an unpaid position. PCM is supported by an MSK core grant (P30CA008748). PGR, AN, EGR, PCM, MB, JYL, RM, DHH, JCR, RFA, CD, MAR, AC, LR, and EC are supported in part by National Cancer Institute-sponsored AIDS Malignancy Consortium grant UM1CA121947 and UM1CA181255. RFA is supported by John Hopkins Cancer Center (5P30CA006973). CB and NM are supported in part by Lymphoma Study Association, and DC and YT are supported in part by the French Agency for Research on AIDS and Viral Hepatitis. DC also has contracts with Jansen and speaker bureau funding from Gilead and Pfizer. All other authors declare no competing interests.
Figures

Comment in
-
Optimising treatment of HIV-associated Hodgkin lymphoma.Lancet Haematol. 2023 Aug;10(8):e563-e564. doi: 10.1016/S2352-3026(23)00177-1. Lancet Haematol. 2023. PMID: 37532413 No abstract available.
References
-
- Olszewski, Adam J; Castillo, Jorge J. Outcomes of HIV-associated Hodgkin lymphoma in the era of antiretroviral therapy. AIDS. 2016;30:787–796. - PubMed
-
- Levine AM, Li P, et al. Chemotherapy consisting of doxorubicin, bleomycin, vinblastine, and dacarbazine with granulocyte-colony-stimulating factor in HIV-infected patients with newly diagnosed Hodgkin’s disease: a prospective, multi-institutional AIDS clinical trials group study (ACTG 149). J Acquir Immune Defic Syndr. 2000; 24(5): 444–50. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

