Macropolyhedral syn-B18H22, the "Forgotten" Isomer
- PMID: 37532522
- PMCID: PMC10436279
- DOI: 10.1021/jacs.3c05530
Macropolyhedral syn-B18H22, the "Forgotten" Isomer
Abstract
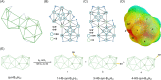
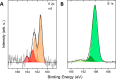
The chemistry and physics of macropolyhedral B18H22 clusters have attracted significant attention due to the interesting photophysical properties of anti-B18H22 (blue emission, laser properties) and related potential applications. We have focused our attention on the "forgotten" syn-B18H22 isomer, which has received very little attention since its discovery compared to its anti-B18H22 isomer, presumably because numerous studies have reported this isomer as nonluminescent. In our study, we show that in crystalline form, syn-B18H22 exhibits blue fluorescence and becomes phosphorescent when substituted at various positions on the cluster, associated with peculiar microstructural-dependent effects. This work is a combined theoretical and experimental investigation that includes the synthesis, separation, structural characterization, and first elucidation of the photophysical properties of three different monothiol-substituted cluster isomers, [1-HS-syn-B18H21] 1, [3-HS-syn-B18H21] 3, and [4-HS-syn-B18H21] 4, of which isomers 1 and 4 have been proved to exist in two different polymorphic forms. All of these newly substituted macropolyhedral cluster derivatives (1, 3, and 4) have been fully characterized by NMR spectroscopy, mass spectrometry, single-crystal X-ray diffraction, IR spectroscopy, and luminescence spectroscopy. This study also presents the first report on the mechanochromic shift in the luminescence of a borane cluster and generally enriches the area of rather rare boron-based luminescent materials. In addition, we present the first results proving that they are useful constituents of carbon-free self-assembled monolayers.
Conflict of interest statement
The authors declare no competing financial interest.
Figures









References
-
- Horsky T. N.; Hahto S. K.; McIntyre E. K.; Sacco G. P.; Matsuo J.; Kase M.; Aoki T.; Seki T.. N- and P-Type Cluster Source; Kyoto, (Japan) 2011, 452–−455.10.1063/1.3548447. - DOI
-
- Dash B. P.; Satapathy R.; Maguire J. A.; Hosmane N. S. Polyhedral Boron Clusters in Materials Science. New J. Chem. 2011, 35, 1955.10.1039/c1nj20228f. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

