Genetic insights into resting heart rate and its role in cardiovascular disease
- PMID: 37532724
- PMCID: PMC10397318
- DOI: 10.1038/s41467-023-39521-2
Genetic insights into resting heart rate and its role in cardiovascular disease
Abstract
Resting heart rate is associated with cardiovascular diseases and mortality in observational and Mendelian randomization studies. The aims of this study are to extend the number of resting heart rate associated genetic variants and to obtain further insights in resting heart rate biology and its clinical consequences. A genome-wide meta-analysis of 100 studies in up to 835,465 individuals reveals 493 independent genetic variants in 352 loci, including 68 genetic variants outside previously identified resting heart rate associated loci. We prioritize 670 genes and in silico annotations point to their enrichment in cardiomyocytes and provide insights in their ECG signature. Two-sample Mendelian randomization analyses indicate that higher genetically predicted resting heart rate increases risk of dilated cardiomyopathy, but decreases risk of developing atrial fibrillation, ischemic stroke, and cardio-embolic stroke. We do not find evidence for a linear or non-linear genetic association between resting heart rate and all-cause mortality in contrast to our previous Mendelian randomization study. Systematic alteration of key differences between the current and previous Mendelian randomization study indicates that the most likely cause of the discrepancy between these studies arises from false positive findings in previous one-sample MR analyses caused by weak-instrument bias at lower P-value thresholds. The results extend our understanding of resting heart rate biology and give additional insights in its role in cardiovascular disease development.
© 2023. The Author(s).
Conflict of interest statement
N.V. is currently employed at Regeneron plc. The 23andMe Research team members are current or former employees of 23andMe, Inc. and hold stock or stock options in 23andMe. P. Sever has received research awards from Pfizer Inc. I.N. is now a full-time employee at Gilead. B.M.P. serves on the Steering Committee of the Yale Open Data Access Project funded by Johnson & Johnson. K.S., H.H., D.F.G., D.O.A., and G.S. are employees of deCODE genetics/Amgen Inc. C.R. is currently employed at the Broad institute and is supported by a grant from Bayer AG to the Broad Institute focused on the development of therapeutics for cardiovascular disease. M.L. receives consulting or lecturing fees from Amgen, Astellas, UCB, Consilient Health, GE/Lunar, Tromp/Hologic, Renapharma, Meda/Mylan, Janssen-Cilag and Radius Health. W.M. is employed with Synlab Holding Deutschland GmbH. M.E.K. is employed with Synlab Holding Deutschland GmbH and reports grants and personal fees from AMGEN, BASF, Sanofi, Siemens Diagnostics, Aegerion Pharmaceuticals, Astrazeneca, Danone Research, Numares, Pfizer, Hoffmann LaRoche; personal fees from MSD, Alexion; grants from Abbott Diagnostics, all outside the submitted work. The remaining authors declare no competing interests.
Figures
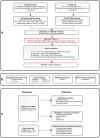


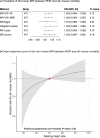


References
-
- Jouven X, et al. Heart-rate profile during exercise as a predictor of sudden death. N. Engl. J. Med. 2005;352:1951–1958. - PubMed
-
- Lonn EM, et al. Heart rate is associated with increased risk of major cardiovascular events, cardiovascular and all-cause death in patients with stable chronic cardiovascular disease: an analysis of ONTARGET/TRANSCEND. Clin. Res. Cardiol. 2014;103:149–159. - PubMed
Publication types
MeSH terms
Grants and funding
- MC_UU_00007/10/MRC_/Medical Research Council/United Kingdom
- MC_PC_U127592696/MRC_/Medical Research Council/United Kingdom
- MC_UU_00006/1/MRC_/Medical Research Council/United Kingdom
- MR/R010676/1/MRC_/Medical Research Council/United Kingdom
- 100114/WT_/Wellcome Trust/United Kingdom
- G9815508/MRC_/Medical Research Council/United Kingdom
- 217065/Z/19/Z/WT_/Wellcome Trust/United Kingdom
- MC_PC_19009/MRC_/Medical Research Council/United Kingdom
- 212945/Z/18/Z/WT_/Wellcome Trust/United Kingdom
- R01 DK072193/DK/NIDDK NIH HHS/United States
- MC_UU_00011/1/MRC_/Medical Research Council/United Kingdom
- C18281/A29019/CRUK_/Cancer Research UK/United Kingdom
- MC_UU_00002/7/MRC_/Medical Research Council/United Kingdom
- R01 DK062370/DK/NIDDK NIH HHS/United States
- R01 HL105756/HL/NHLBI NIH HHS/United States
- MC_PC_15018/MRC_/Medical Research Council/United Kingdom
- 202802/Z/16/Z/WT_/Wellcome Trust/United Kingdom
- MC_UU_00032/1/MRC_/Medical Research Council/United Kingdom
- 204623/Z/16/Z/WT_/Wellcome Trust/United Kingdom

