Effect of nanostructure lipid carrier of methylene blue and monoterpenes as enzymes inhibitor for Culex pipiens
- PMID: 37532732
- PMCID: PMC10397322
- DOI: 10.1038/s41598-023-39385-y
Effect of nanostructure lipid carrier of methylene blue and monoterpenes as enzymes inhibitor for Culex pipiens
Abstract
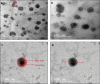
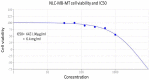
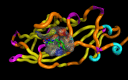
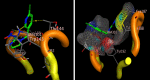
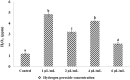
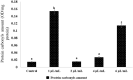
Solid lipid nanoparticles second generation, nanostructure lipid carrier (NLC), is one of the most important biodegradable nanoparticles. Nanostructure Lipid carrier (NLC) was used to encapsulate methylene blue (MB) dye, carvacrol and citronellal and their efficacy as insecticidal against Culex pipiens (Cx. pipiens) were distinguished. The prepared nanoformulation revealed very good physicochemical properties, especially the homogeneity of the particle size. Transmission electron microscope showed spherical shaped nanoparticles within range less than 200 nm. The prepared NLC-MB-MT system showed a very competitive insecticidal activity and high virulence against the mosquito larvae with higher mortality rate of LC50 of 0.141 µl/mL, in addition to high level of Oxidative stress parameters obtained through all the tested enzymes including hydrogen peroxide (4.8 ppm), protein carbonyl amount (0.12 OD/mg protein), ascorbic acid (0.15 mg) and Superoxide dismutase (SOD) showed strong increasing (0.09 OD/mg protein/min) at 6 µg/mL, respectively. Whereas paradoxical results of the oxidative stress enzymes were obtained from different concentration of nanoformulation that introduce a convenient reason for their potential insecticidal effect. The cytotoxic effect of NLC-MB-MT was evaluated using WI38 human lung cell lines, the LC50 was 6.4 mg/mL. The low cytotoxic reactivity towards the tested cell line makes the NLC-MB-MT nanoformulation has its promising insecticidal efficacy. Molecular docking study for each component were done against acetylcholine esterase protein and accepted binding modes achieved by the three compounds.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

