Consent document translation expense hinders inclusive clinical trial enrolment
- PMID: 37532930
- PMCID: PMC11046417
- DOI: 10.1038/s41586-023-06382-0
Consent document translation expense hinders inclusive clinical trial enrolment
Abstract
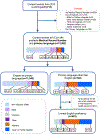
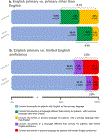
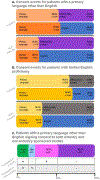
Patients from historically under-represented racial and ethnic groups are enrolled in cancer clinical trials at disproportionately low rates in the USA1-3. As these patients often have limited English proficiency4-7, we hypothesized that one barrier to their inclusion is the cost to investigators of translating consent documents. To test this hypothesis, we evaluated more than 12,000 consent events at a large cancer centre and assessed whether patients requiring translated consent documents would sign consent documents less frequently in studies lacking industry sponsorship (for which the principal investigator pays the translation costs) than for industry-sponsored studies (for which the translation costs are covered by the sponsor). Here we show that the proportion of consent events for patients with limited English proficiency in studies not sponsored by industry was approximately half of that seen in industry-sponsored studies. We also show that among those signing consent documents, the proportion of consent documents translated into the patient's primary language in studies without industry sponsorship was approximately half of that seen in industry-sponsored studies. The results suggest that the cost of consent document translation in trials not sponsored by industry could be a potentially modifiable barrier to the inclusion of patients with limited English proficiency.
© 2023. The Author(s), under exclusive licence to Springer Nature Limited.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

